The following plot results from interim data disclosed at a press conference and reported by The New York Times. The data is from a subset of a Phase 3 clinical trial in progress in Turkey. My thanks to Kyle Felker (Argonne National Lab), who spotted the data.
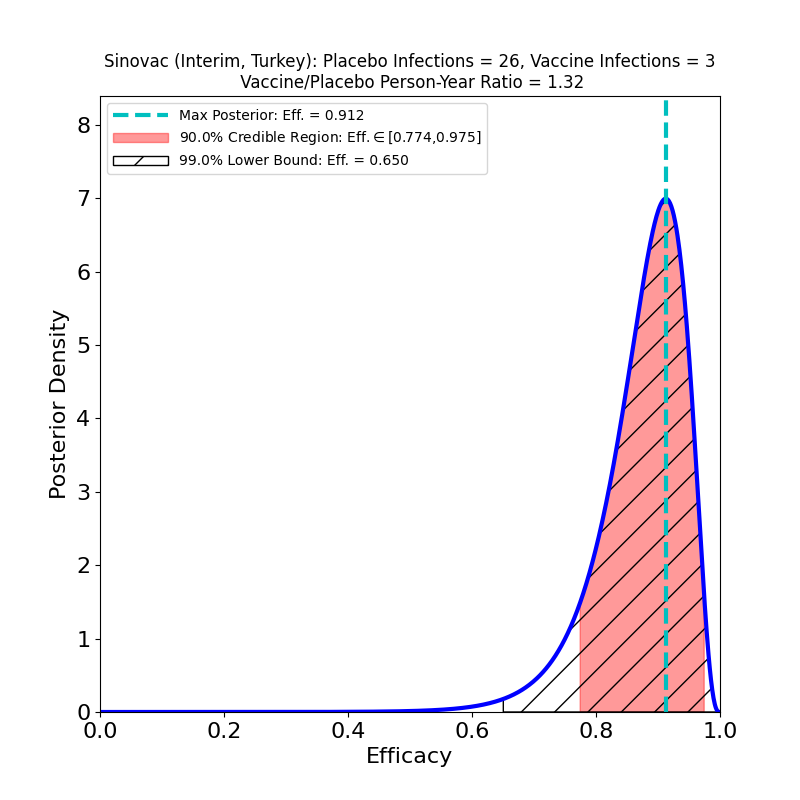
The analysis looks encouraging, with a peak-posterior efficacy of 91%. With these small numbers, that efficacy is not particularly well-constrained, however, as can be seen from the plot — the lower bound of the 90% credible region is 0.77, and the 99% lower bound is 0.65. This kind of spread is to be expected for an interim result, and hopefully the results will firm up soon with disclosure of the full clinical trial results.