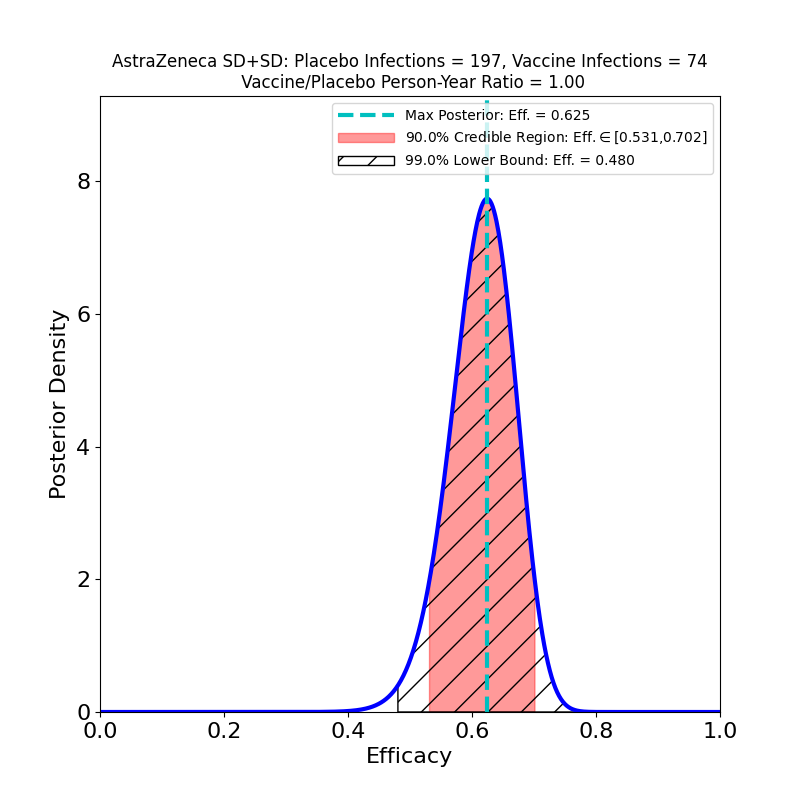
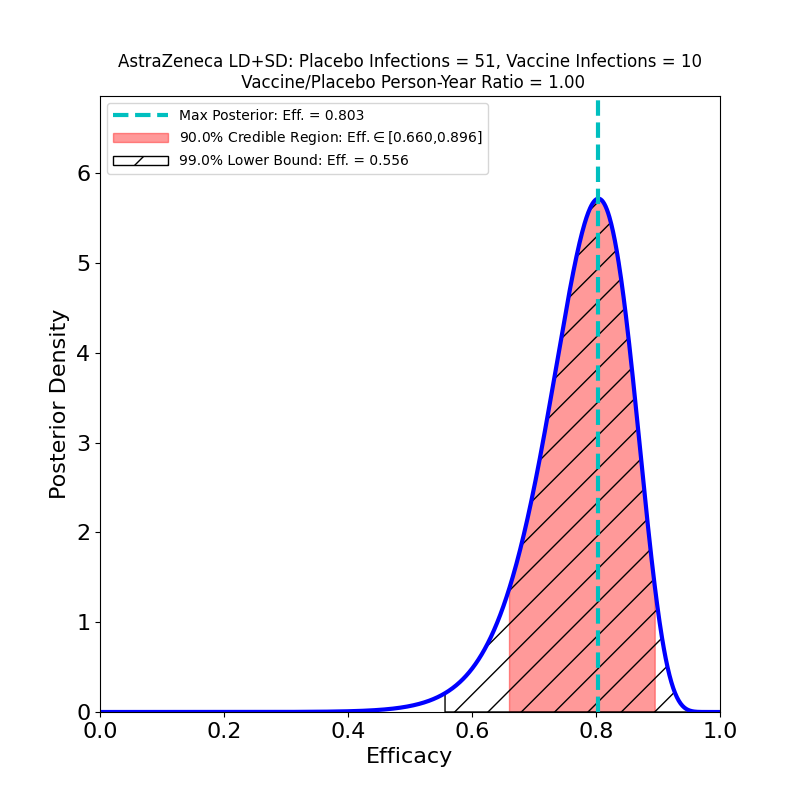
These plots result from the analysis of the Phase 3 trials of AZ’s 2-dose adenovirus-based vaccine, reported in this article. Note that AZ kind of botched this trial, administering the wrong 1st dose (50% of planned standard dose) to a subset of participants. In so doing they stumbled into a serendipitous discovery: the efficacy of a low-dose followed by a standard-dose booster is higher than the efficacy of the originally-planned two-standard-dose vaccination.
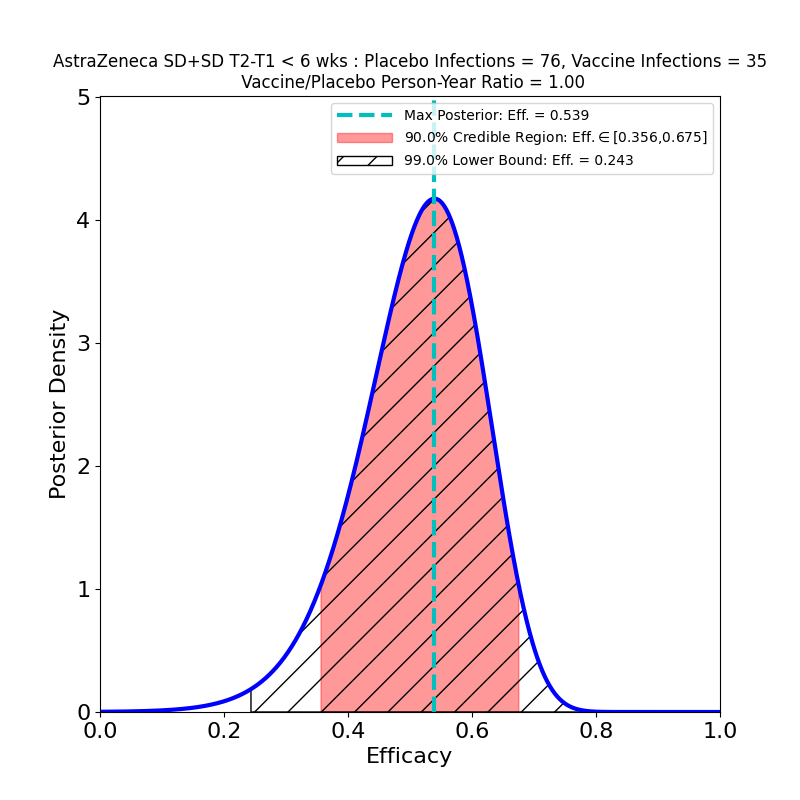
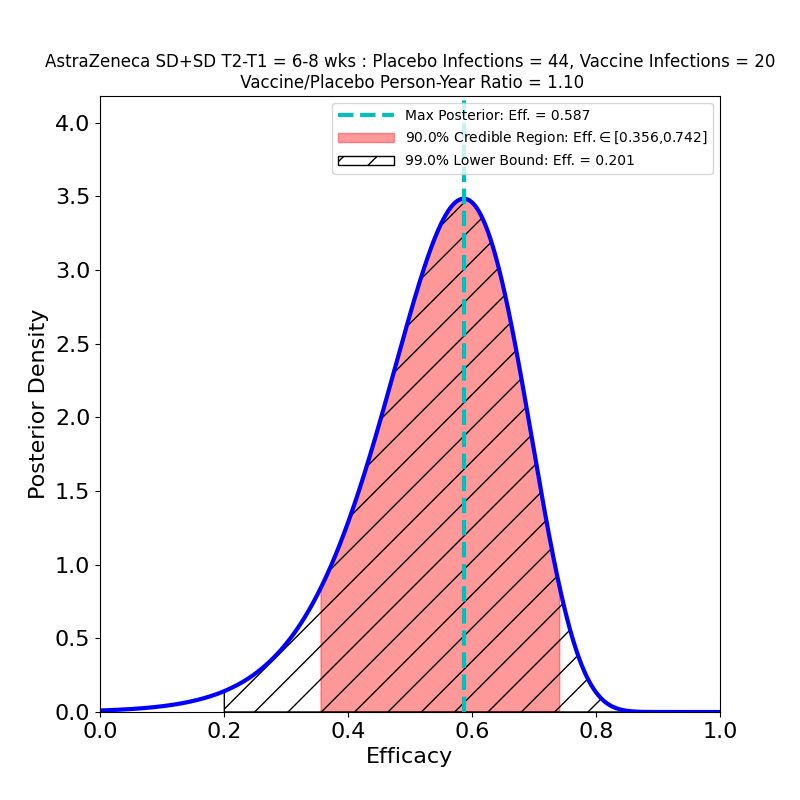
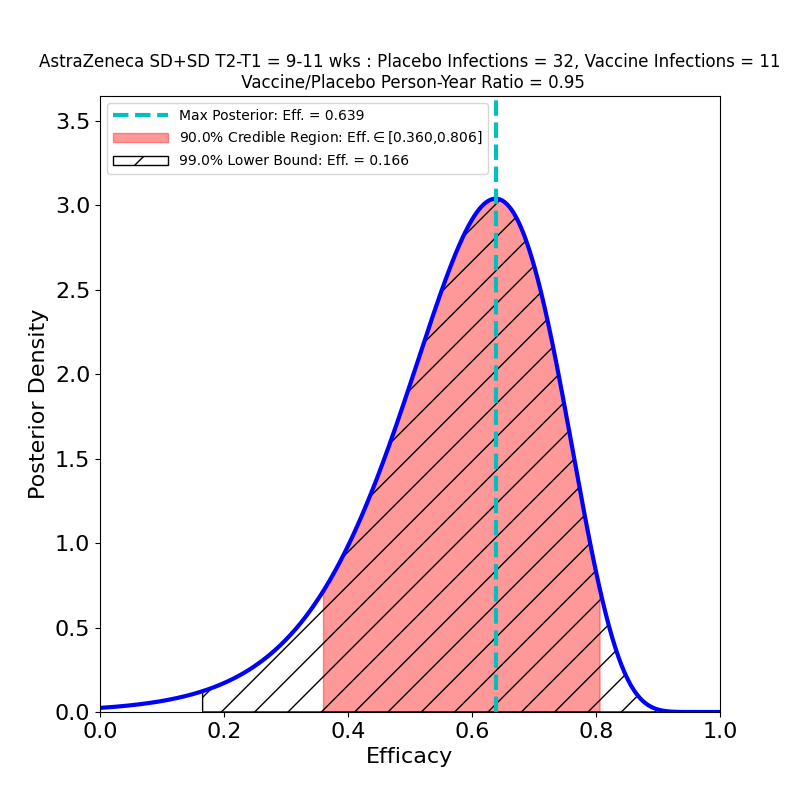
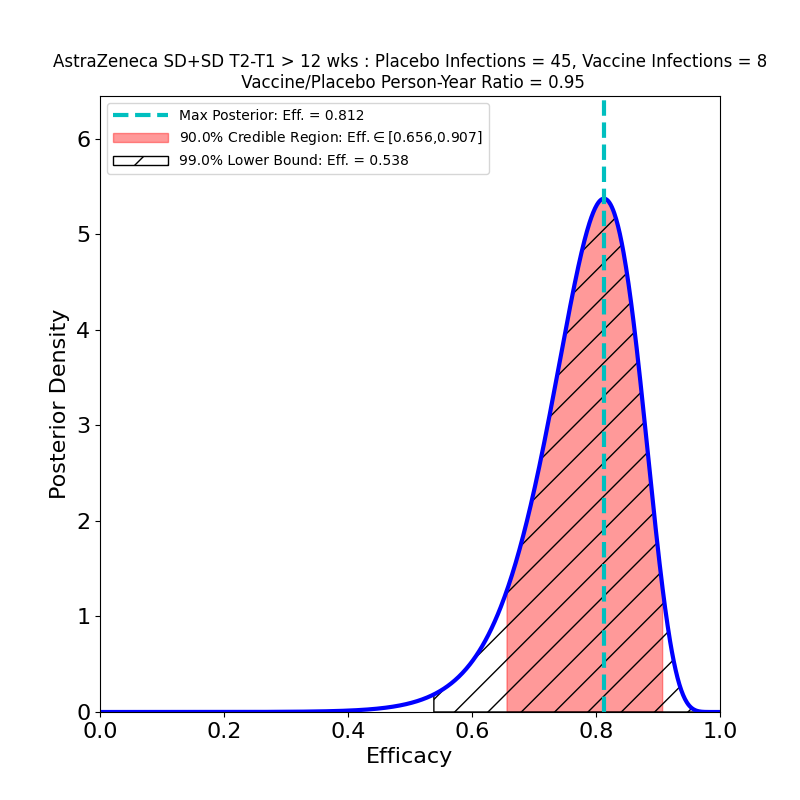
The overall story for SD+SD is not as bad as it appears, however. The authors broke out the numbers by time elapsed between shot 1 and shot 2. Again, it seems like a strange study “design” that this parameter should be a variable, and suggests a rather improvised design. The authors of the article assert that this variability in dose schedule was due to manufacturing delays. Once again they succeeded in making lemonade from lemons, as this feature of the clinical study allowed them to see the evolution of efficacy with booster shot delay time:
These plots are for protective efficacy against “symptomatic” COVID-19. One can clearly see a progression here, with efficacy growing as the time-delay of the booster shot increases, and with final efficacy comparable to the “LD+SD” results above.
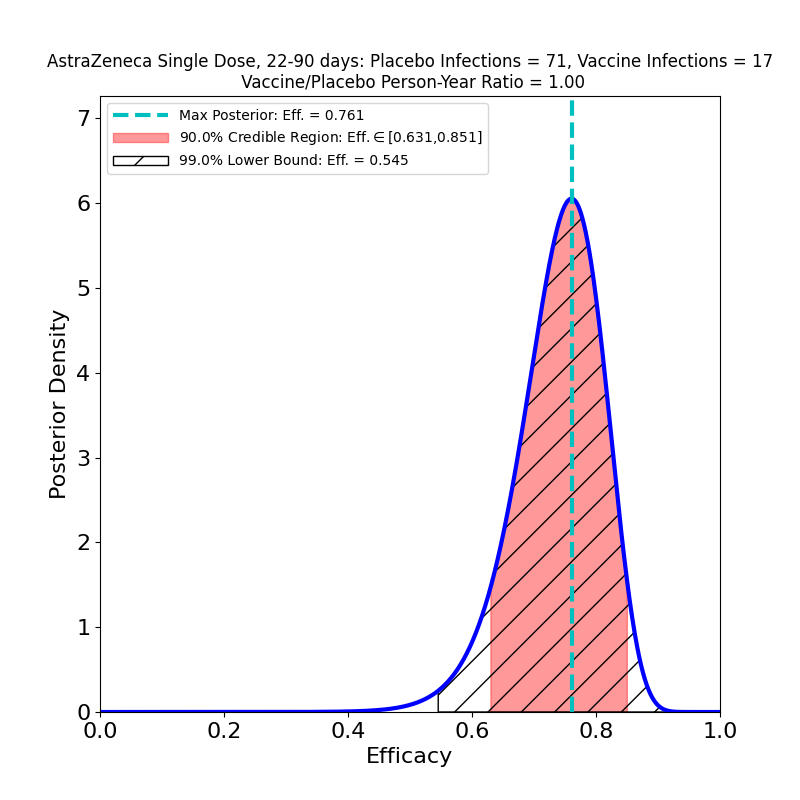
The article also reports “single-shot” data from study subjects who declined to have a second shot after having received the first shot (at standard dosage, i.e. “SD”). The data for all symptomatic COVID-19 cases diagnosed 22-90 days post-vaccination of such single-dose study subjects yields the following results:
A comparison with the “LD+SD” plot above suggests that the protective immunity of the vaccine is about the same — the peak efficacies are close (0.761 vs. 0.803), and the 90% credible regions overlap quite a bit. A comparison with the lower-right panel of the previous figure (SD+SD, booster shot delay > 12 weeks) leads to a similar conclusion — there’s little to choose from between a single SD shot or an SD+SD schedule separated by 12 weeks!
It’s a bit puzzling to sort out all this. Apparently LD+SD, SD+SD spaced out by 12 weeks or more, or a single SD dose all yield about the same efficacy, while SD followed by an SD booster with an insufficient delay actually lowers the protective efficacy of this vaccine with respect to what it would be if the booster were simply omitted!
This is the oddest study of a vaccine that I’ve seen so far. It’s a relief that the evidence for strong efficacy is so clear, of course. But there are serious scientific questions raised by these results that will certainly require further investigation.
And, AZ needs to raise its standards for conducting clinical trials.
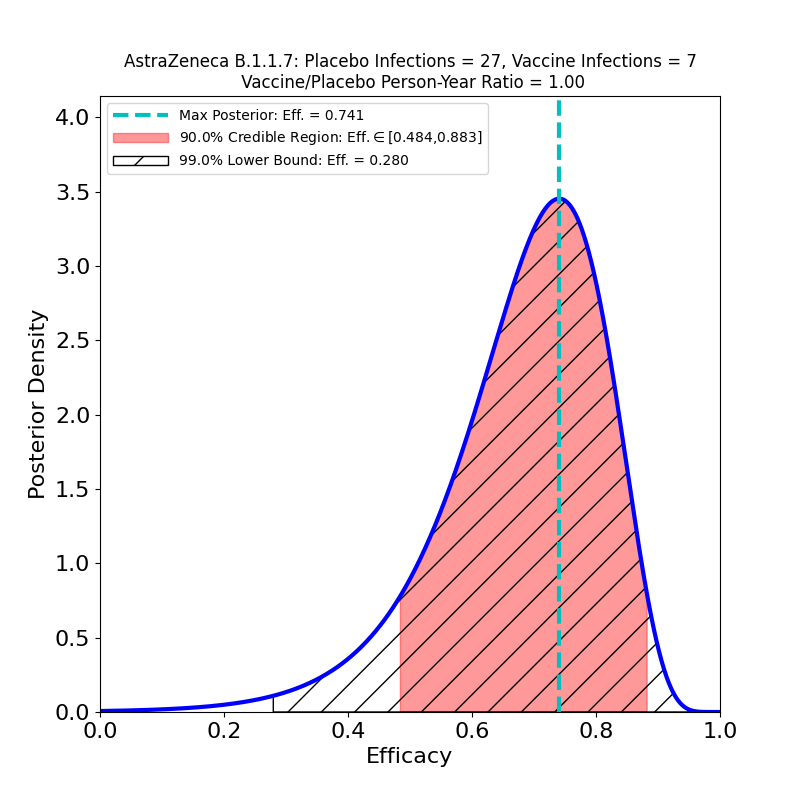
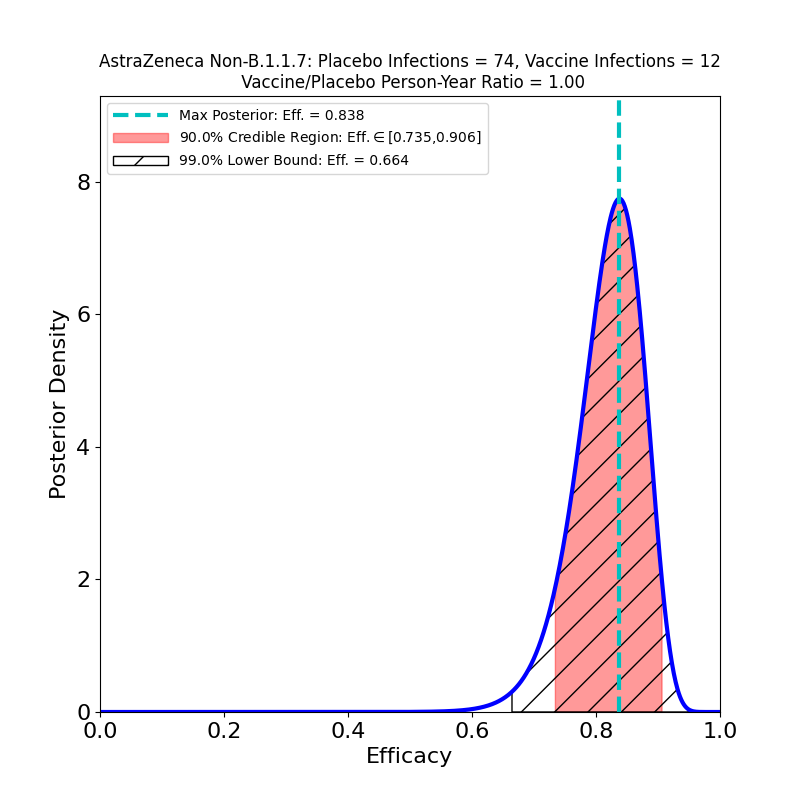
Update, 5 February 2021: A preprint appeared today in The Lancet showing the results of the vaccine trials broken out by virus variant — the B.1.1.7 variant whose prevalence in the UK is increasing (and which has a higher R0 than the “classic” virus strains, see Figure 1b of this paper). The authors sequenced swabs from study participants to separate the B.1.1.7 infections from other infections. Table 1 of the new preprint has the data. The “symptomatic” case data leads to the following Bayesian analysis output:
These data combine both SD+SD and LD+SD cases. On the basis of these plots I would have to say that the claims in the media of the vaccine’s equivalent efficacy against the B.1.1.7 strain are a bit too enthusiastic. The claim is based on a comparison of the most likely efficacy values (74% vs 84%), but takes no account of the uncertainty due to the small sample. Note that according to the 90% credible region the efficacy against B.1.1.7 could be as low as 48%. It is almost certainly higher than that, but note that the probability distribution in the left panel of the figure has considerably more mass to the left of the peak than to its right, meaning that the actual efficacy is quite likely to be lower than 74%.
In fact, the preprint cites a 95% confidence interval (technically a different measure of uncertainty from the credible regions given on this site) of [41.6%,88.9%], so the authors are — as expected — diligent about reporting the uncertainty in the result. It’s unfortunate that these caveats get lost in media reports, however, because on present evidence we should be prepared for the possibility that the AZ vaccine (and other vaccines as well) will be found to have much lower efficacy against B.1.1.7 and other strains — compare the case of the Novavax vaccine.