Pfizer has a press release today concerning its phase 3 trial of its BNT162b2 COVID-19 vaccine in U.S. adolescents, ages 12-15. There’s only one topline number from the trial in the press release — 18 cases in the placebo group, 0 in the vaccine group. The result is the following plot:
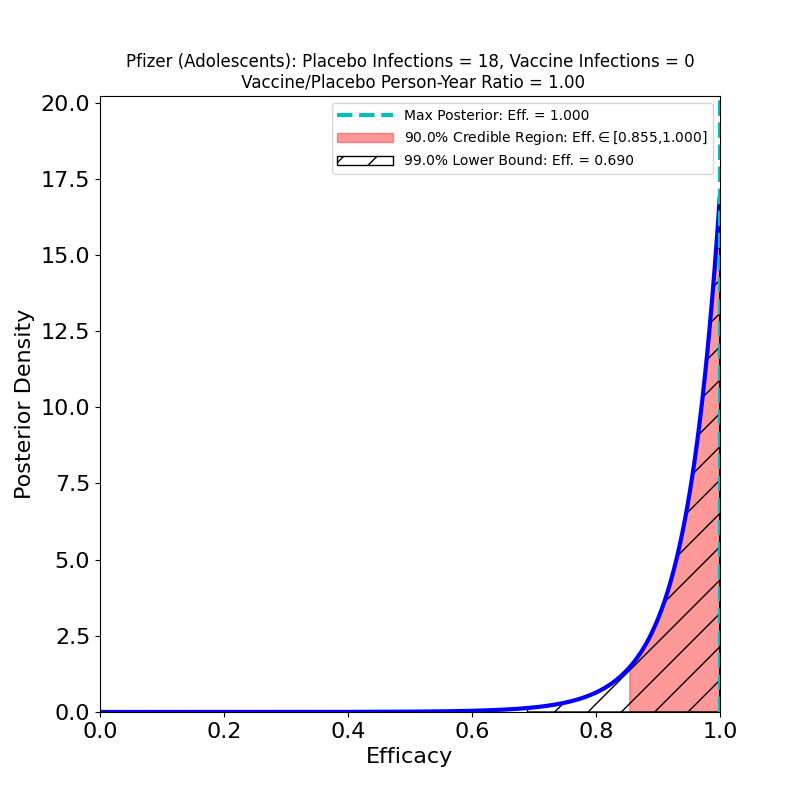
This is a very impressive result — with 90% probability the efficacy in adolescents is higher than 85.5%. It should probably not be regarded as a very surprising result: the immune systems of adolescents are generally better-functioning and more responsive than those of adults, so it would be more surprising if the efficacy were lower than the result found for older individuals. The more relevant part of the study is probably the fact that the vaccine is safe to use even at age 12, which is good news.
A few comments:
(1) The press release does not state the time period over which the study was conducted, so it isn’t possible to assess from this information the extent to which protectiveness extends to variants currently in circulation in the US.
(2) This was a small study — about 2,200 individuals (as opposed to 45,000 in the principal study). For this reason, the efficacy results are less constrained (the curves are broader) for adolescents than for older individuals.
(3) As usual, the fact that there were zero COVID-19 cases in the vaccine group in this small study is misleadingly described as representing “100%” efficacy in the press release, a claim picked up by science news reporters who should know better. From the above plot, a plausible value of the efficacy is certainly in the high 90s, but nothing is 100%. When the vaccine is administered to millions of adolescents (as opposed to the 2200 in this study) there will certainly be a few vaccinated adolescents who contract COVID-19. This will not be a vaccine failure, just an illustration of how small probabilities work.
Pfizer will apply to the FDA for an Emergency Use Authorization “as quickly as possible”. When they submit their information packet — which invariably contains a wealth of information, exceeding even what is later published in refereed journal articles — I’ll mine it for another post.