Novavax issued another press release today, with some partial information concerning the now-completed trials, a Phase 3 trial in the UK, and Phase 2b trial in South Africa. The data is a continuation of the data analyzed in this post, and tells a similar story, although with more abundant numbers (and hence less uncertainty). There is some actual data in the press release, but there are also some efficacy estimates not backed by released data, which I can therefore not evaluate here. As usual, when the Emergency Use Authorization request gets to the FDA, a briefing document will be made public with complete trial information. I will issue another update when that happens.
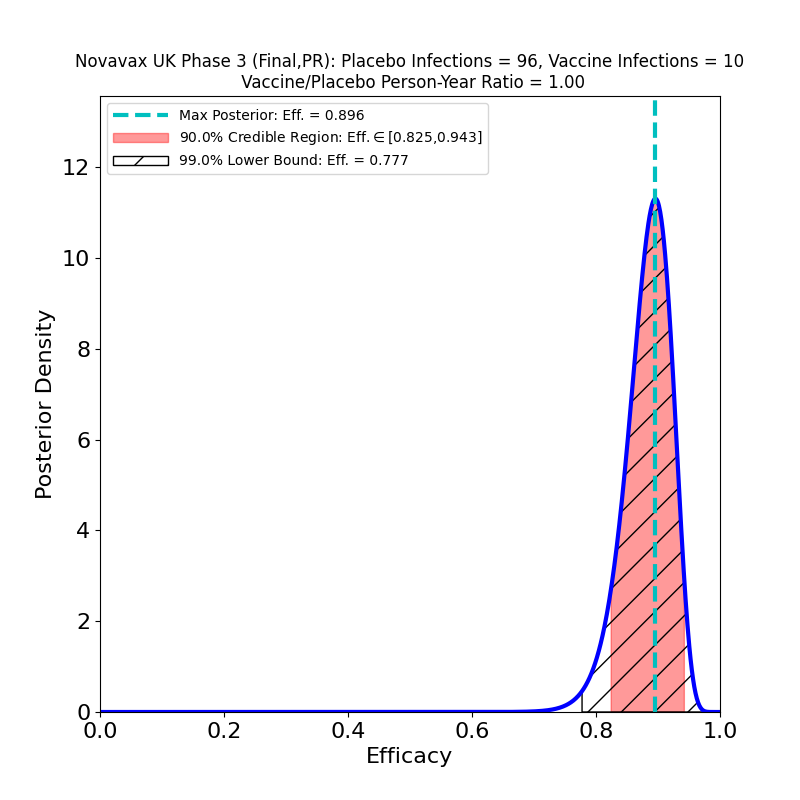
Meantime, here’s what the released data for UK trial looks like:
This looks pretty good. Not that different from the preliminary results, but the constraints on efficacy are tighter, since the numbers are higher. The overall result in the left panel is extremely good (most likely value 90%), despite high prevalence of the B.1.1.7 variant. That’s the variant that is more transmissible, and possibly responsible for more severe disease with worse clinical outcomes, but which is also known not to be a so-called “escape variant” — that is, it is not less susceptible to immunity due to previous infection by “classic” SARS-CoV-2, or due to existing vaccines. So it’s not surprising that the Novavax vaccine stops it.
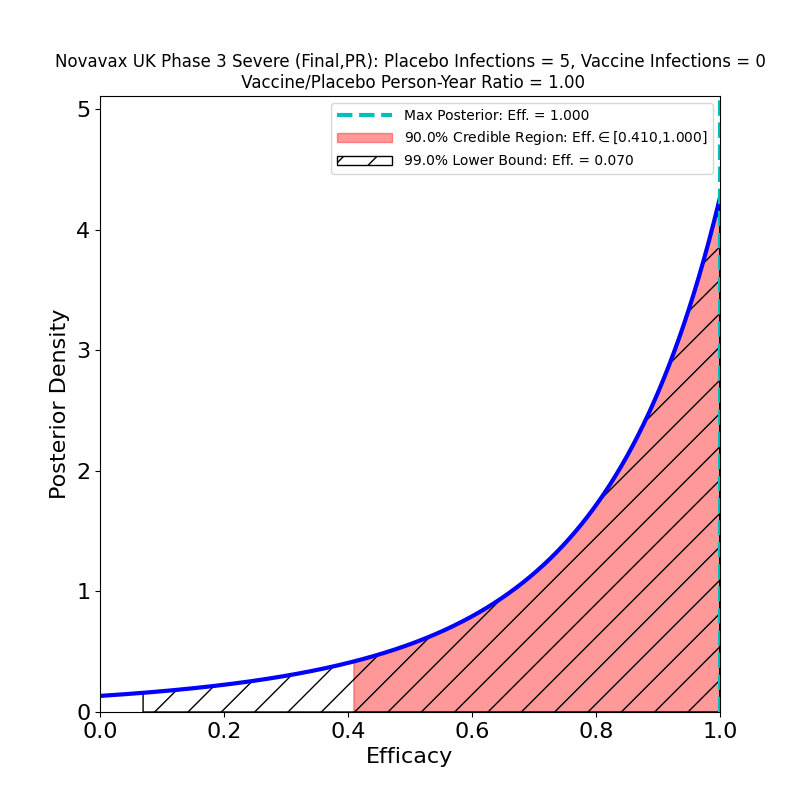
The “severe” disease result is shown on the right. This is what, unfortunately, is described in the press release and reported in naive media outlets as “100%” protection. From the plot it should be clear why this is an inappropriate description of the result — when you have 5 severe cases in the placebo group and 0 in the vaccine group, that’s good news, but nowhere near establishing “100% protection” — based on this data, the 90% credible region says that the protection could be as low as 40%! This kind of thing is the why it is useful to reason in terms of quantified uncertainties, as displayed in these plots, rather than with naive and deceptive point estimates. What we can say based on this data is that there is some moderately weak evidence of efficacy greater than about 40% against severe disease. If Novavax had designed their study as well as Moderna did, including many individuals at risk for severe disease, then they might have collected better evidence (as Moderna did).
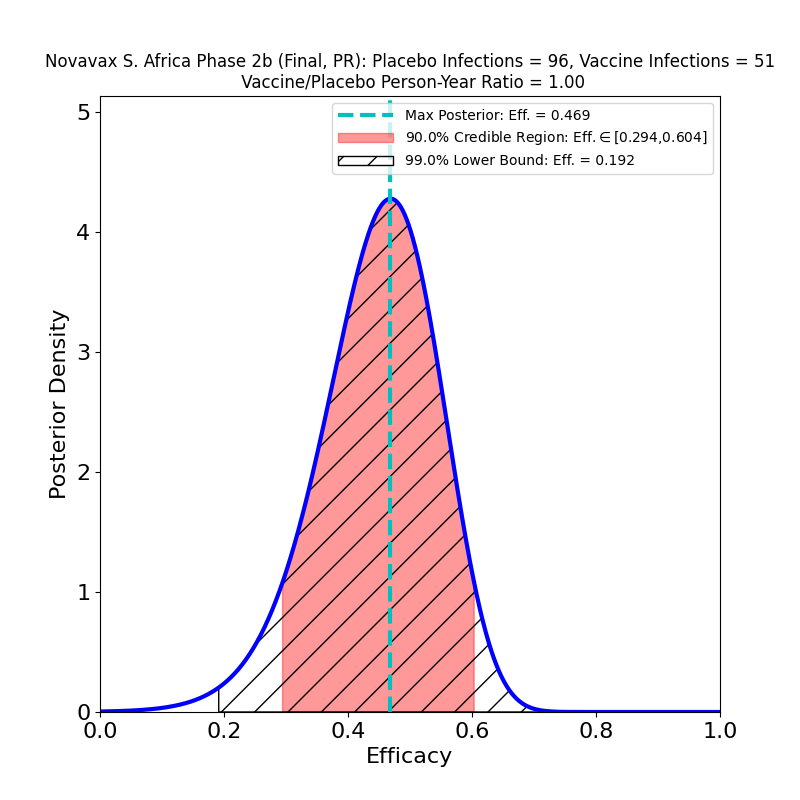
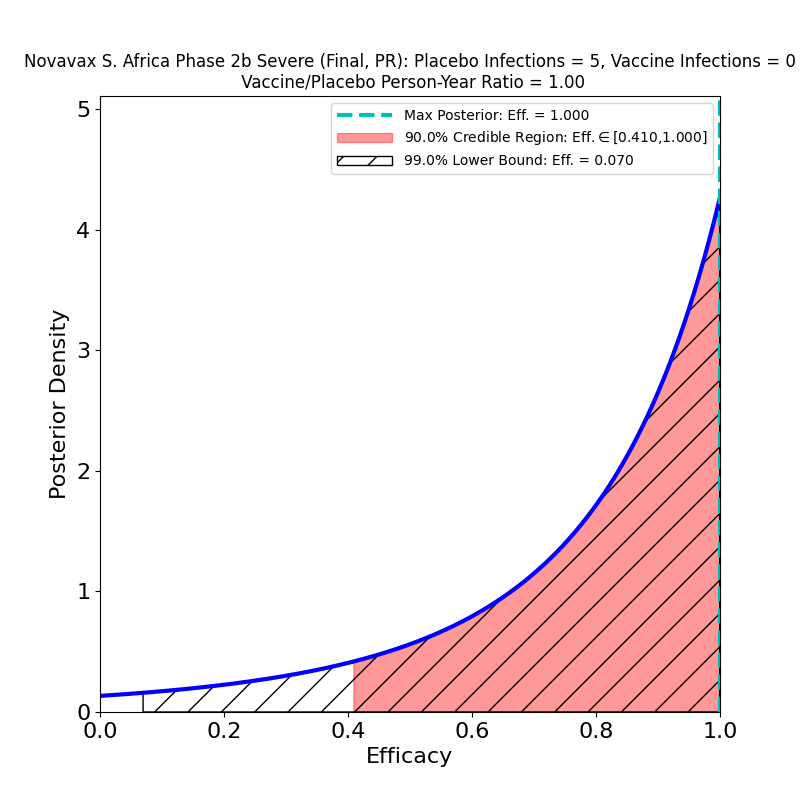
Now, here’s the South African trial data analysis:
In the overall results on the left we see again the troubling effect of the South African “escape variant”, B.1.351, which has already demonstrated its ability to evade immunity produced by the Johnson & Johnson and AstraZeneca vaccines, and for which there is in vitro evidence of decreased immunogenesis by the Pfizer/BioNTech and Moderna vaccines. This result is no worse than the J&J South Africa result, but it’s not any better either. Despite the spin in the press release, it suggests that efficacy to the B.1.351 variant is likely lower than the 50% threshold limit established by the FDA for usefulness. And if the vaccines are this poor at stopping disease, they are almost certainly nearly ineffective at stopping the spread of the virus, since it is being propagated by all all the carriers who get mild or moderate forms of the disease, to say nothing of all the asymptomatic carriers.
This reinforces the point that I made in this post: The B.1.351 variant very likely largely escapes immunity from all existing vaccines. Since (1) we can expect most of the Northern hemisphere to be vaccinated by early summer, so that other variants will be under control, and (2) we know that it only takes a few months for SARS-CoV-2 to travel all over the globe (witness this time it took for the epidemic to spread from Wuhan to the rest of the world in the first place), we can expect that most COVID-19 in the Northern hemisphere will be caused by B.1.351 by sometime this year. Booster vaccines that address B.1.351 are already being prepared — Moderna’s is already in Phase 2, and there’s no doubt that the other manufacturers aren’t far behind. So, we should all get shots as soon as they are available, because they are effective at stopping the spread of classic SARS-CoV-2 and most of its variants. But we should all be prepared to get booster shots this year, as soon as the B.1.351-specific vaccines are available.