Yesterday I updated the post on the Pfizer/BioNTech vaccine in light of a letter to NEJM by D. Skowronski and G. De Serres, in which they showed that in data from Pfizer’s submitted briefing to the FDA for Emergency Use Authorization there is information that can shed light on efficacy of a single dose of the vaccine. Go take a quick look at the post, then come back.
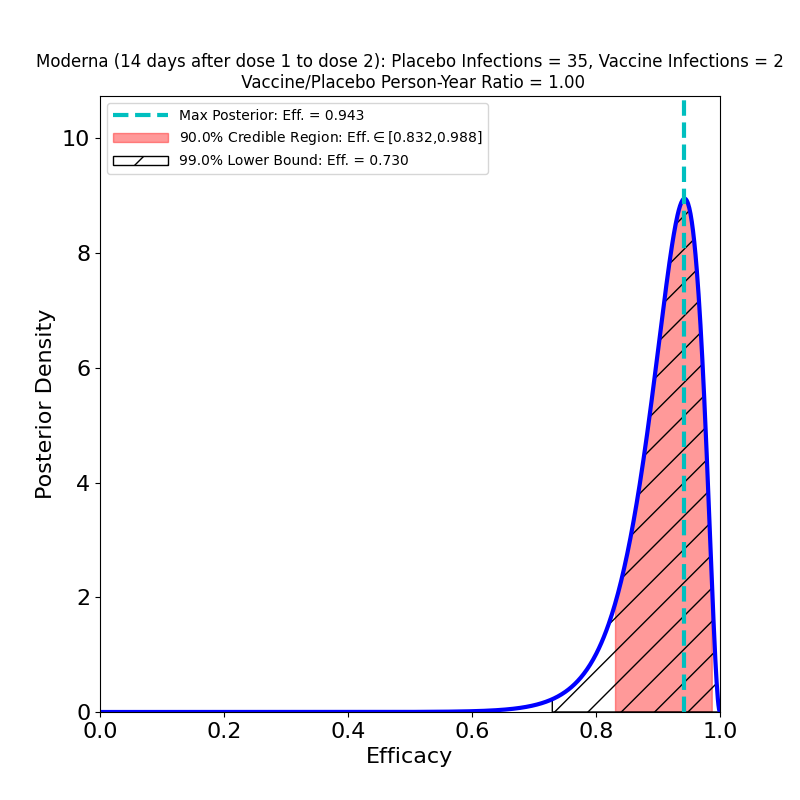
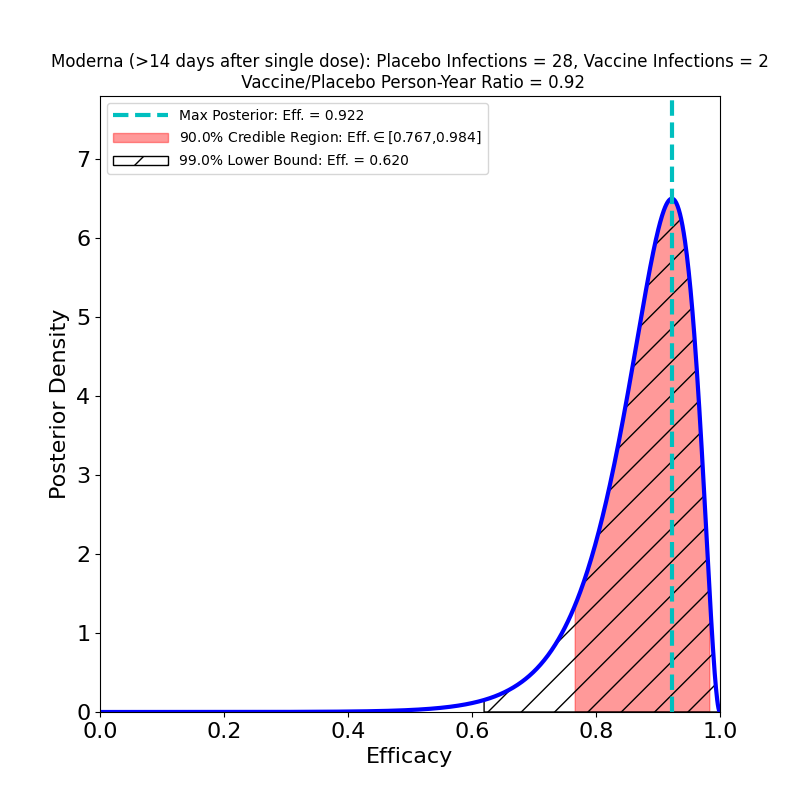
OK, it turns out that you can do the same cool hack on Moderna’s vaccine data. In the NEJM journal article describing the Moderna clinical trial, there’s a table in Figure 3 that lists cases 14 days after dose 1, but before dose 2 (2 in the vaccine group, 28 in the placebo group). Moreover, in Moderna’s EUA submission to the FDA, in Table 15 on p. 28, there’s data on trial subjects who only received 1 dose and contracted COVID-19 14 days or more after vaccination (2/983 in the vaccine group, 28/1059 in the placebo group). In our Bayesian setting, those data look like this:
So, it isn’t just the Pfizer/BioNTech vaccine that creates early immunity: the same is true of Moderna — in fact, the most likely value of the efficacy for the left-hand case (the 2-dose case) is the same as the final top-line value of 94%, whereas in the single-dose case on the right it’s “only” 90%, but the credible region is consistent with the final value, and it’s lower bound is a very respectable 77%!
The 1-dose participants form a less-controlled cohort. In particular, the briefing document notes that the follow-up time during which they were monitored was more heterogeneous (range 1-108 days, median 28 days). Nonetheless this data does extend quite a bit beyond the shot 1-shot 2 time interval, and appears to me to be stronger evidence that a delay of the second shot might be an acceptable choice in face of the vaccine scarcity crisis.
However, it is worth reading again what Aaron Esser-Kahn said in the Pfizer post: there’s a risk that needs to be taken seriously, which is that a weak immune response to immunization in a largish population could encourage viral selection — mutation of resistant variants. So it’s by no means an easy decision. I’m glad it’s not mine to make.