The FDA Emergency Use Authorization (EUA) committe that will decide whether to approve the J&J/Janssen vaccine meets Friday, 26 February 2021, and as promised, the FDA has released the briefing document submitted by the company two days in advance. There’s a great deal of information in such documents, often more than appears in the corresponding journal article, when it is published..
The phase 3 clinical trial was conducted in three countries: the US, Brazil, and South Africa. This is interesting because of the different incidences of SARS-CoV-2 variants prevalent in those locations at the time of the trial: differences are informative about protective efficacy against different variants. In addition, the data cut that seems most relevant is distinguishing the overall “Moderate+Severe COVID-19” endpoint from the “Severe COVID-19” endpoint (as usual, one has to read the document in detail to understand the precise definitions of terms such as “Moderate” and “Severe”). So, let’s take a look.
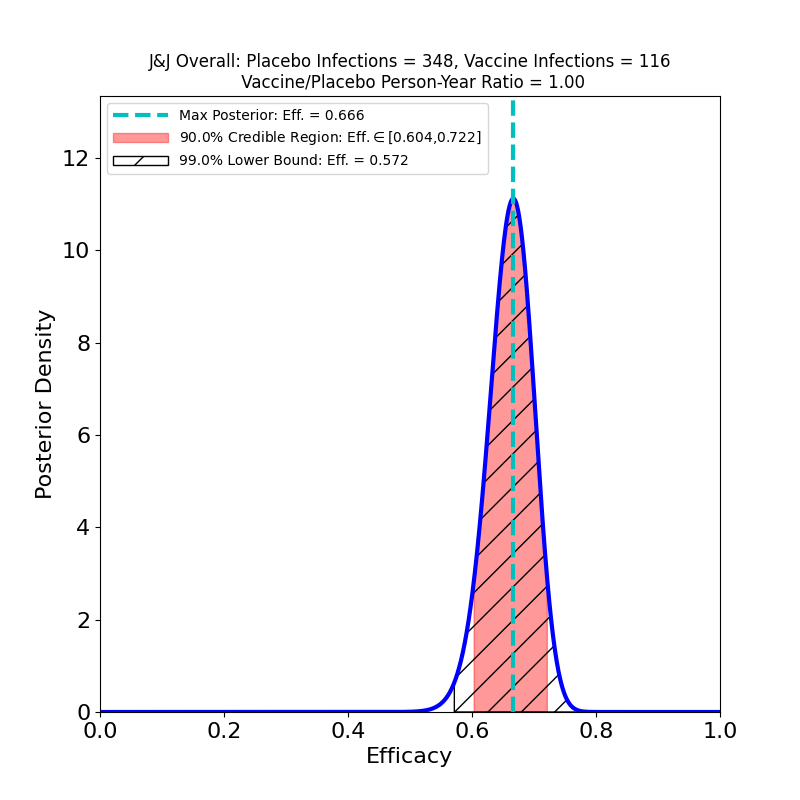
Here’s the top-line efficacy, for “Moderate+Severe” endpoints, across all three countries, infections beginning 14 days post-vaccination.
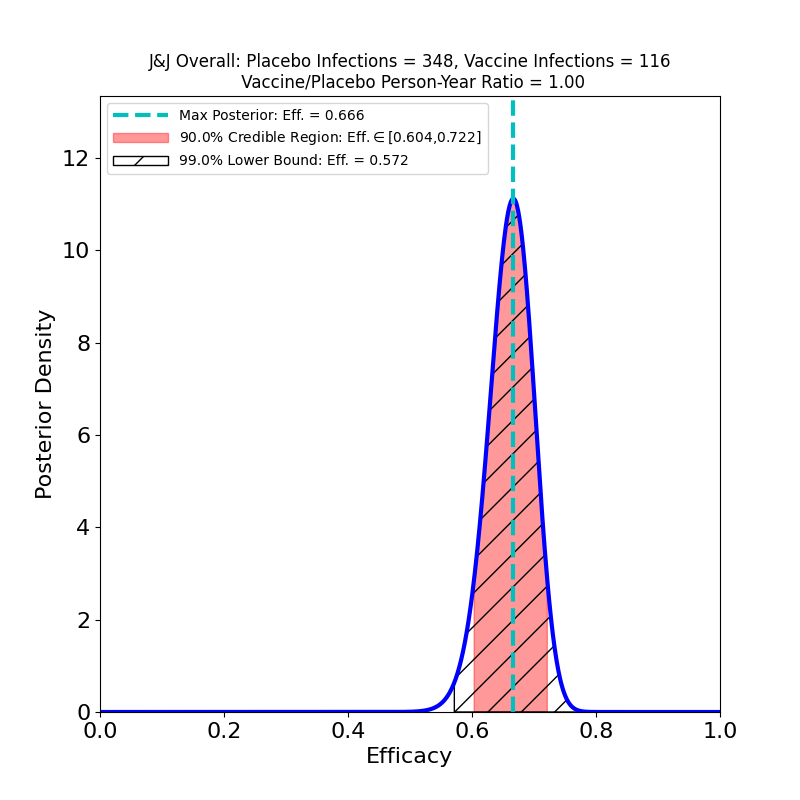
The overall efficacy looks pretty good: 67%, with pretty tight bounds. One should be cautious of invidious comparisons with the crazy-high efficacies of the Moderna and Pfizer/BioNTech vaccines: For an individual, 67% means a 2/3 reduction in the probability of contracting COVID-19 in any interaction with an infectious person. For a vaccinated population, this kind of efficacy means that the virus is in serious trouble: it means that if you could vaccinate everyone with this efficacy, you could get to herd immunity almost immediately (assuming an R0 of 3-ish).
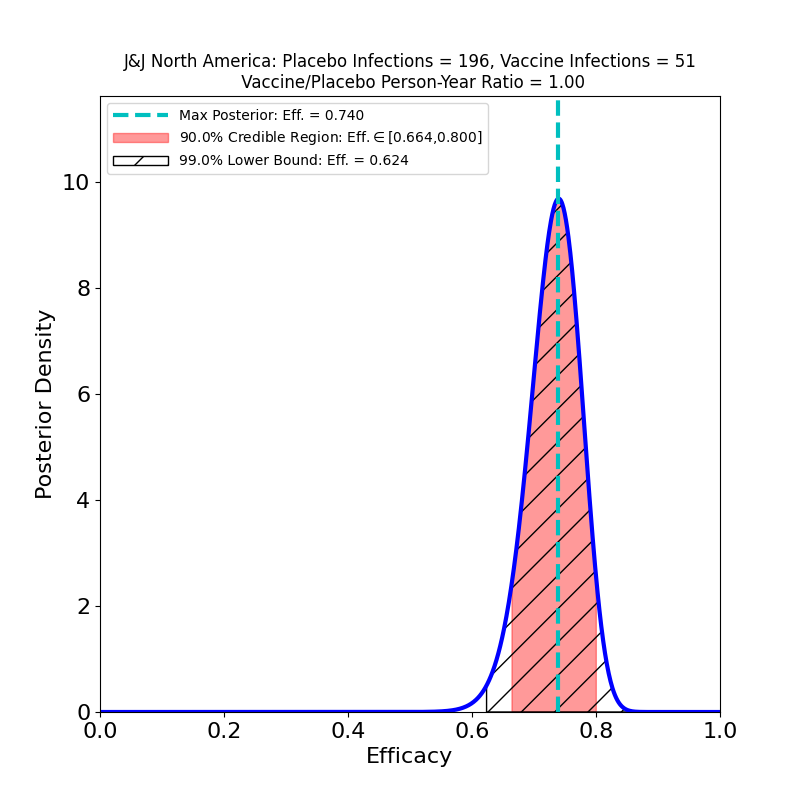
So far so good. Let’s dig into the subgroup analyses. First, here’s protectiveness against “Moderate+Severe” COVID-19 across countries:
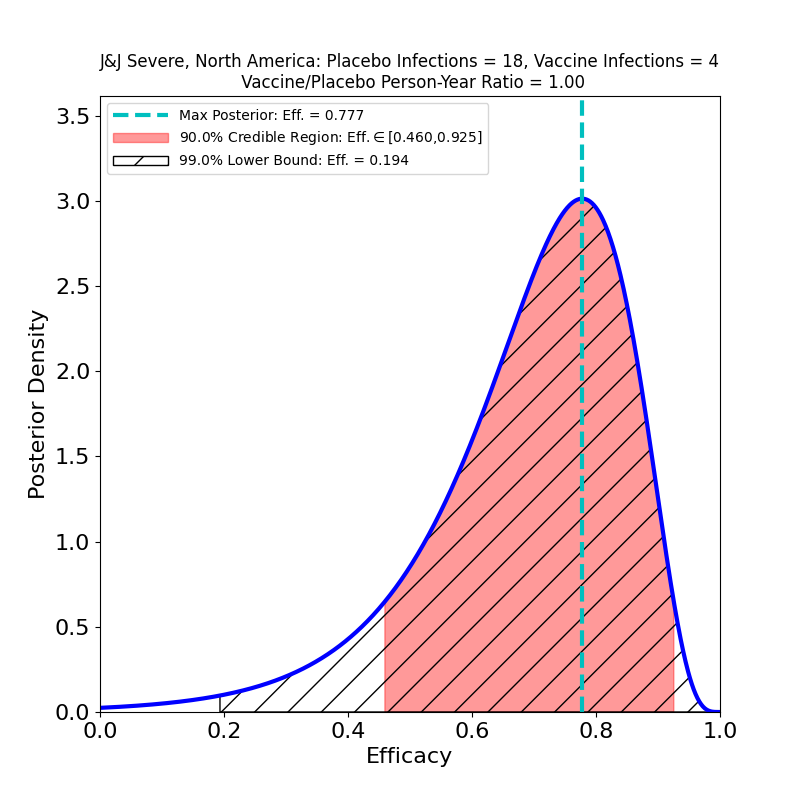
The news here is about variants, and it’s mixed. The efficacy is highest (74% peak value) in the USA, where the circulating variant is principally the Wuhan-H1 variant D614G (“classic” SARS-CoV-2), which was found in 96.4% of sequenced cases in the study.
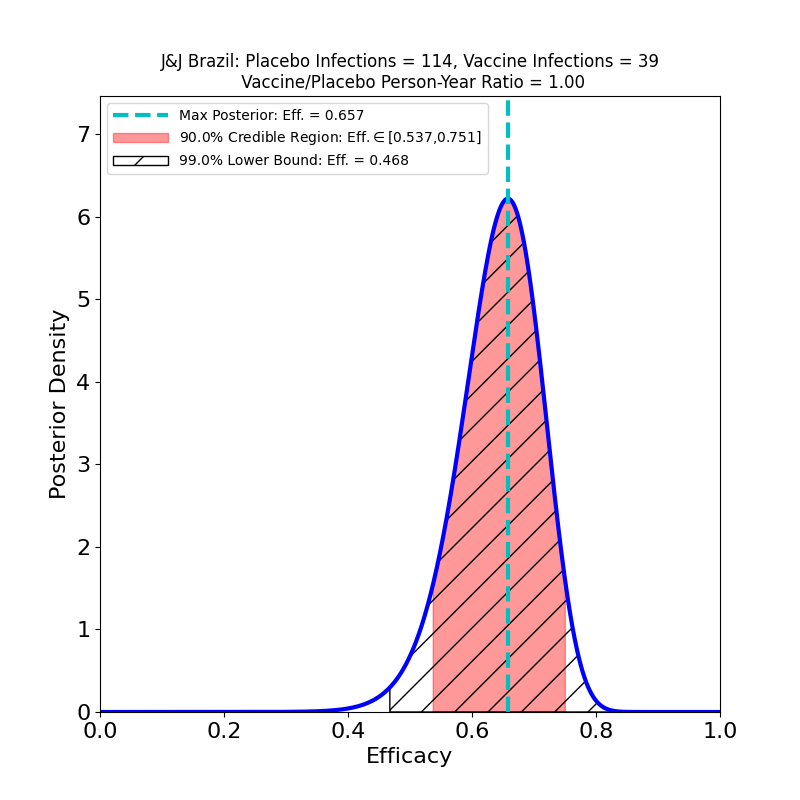
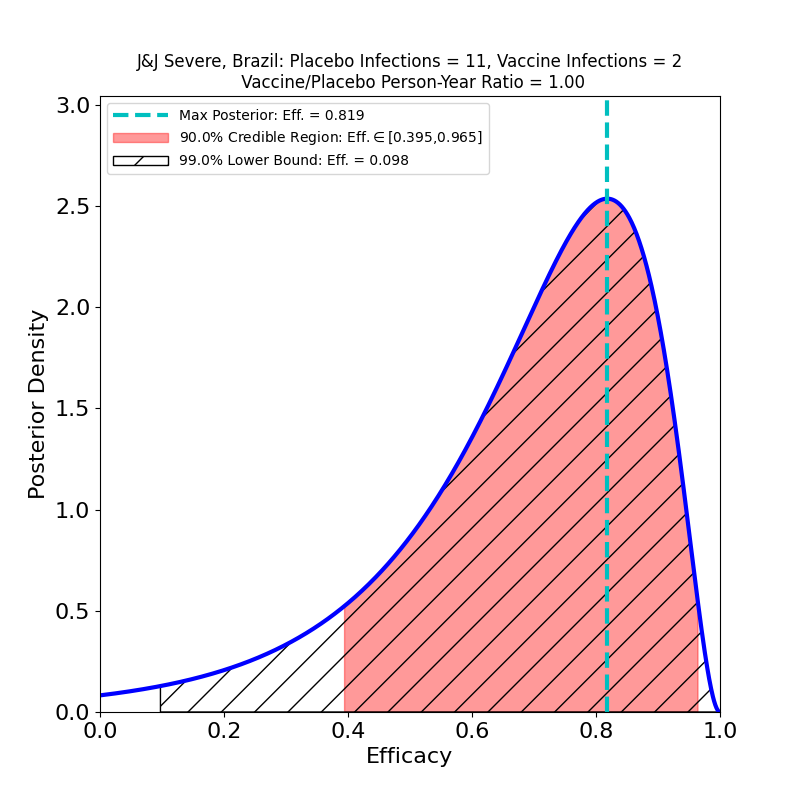
In Brazil, where the study found a mix of P1 lineage (69.4%) and D614G (30.6%) the peak efficacy is a bit lower than in the USA (66%), but the wider curve (due to smaller numbers in the subgroup) shows that it’s quite possible that the efficacy in Brazil is the same as in the USA.
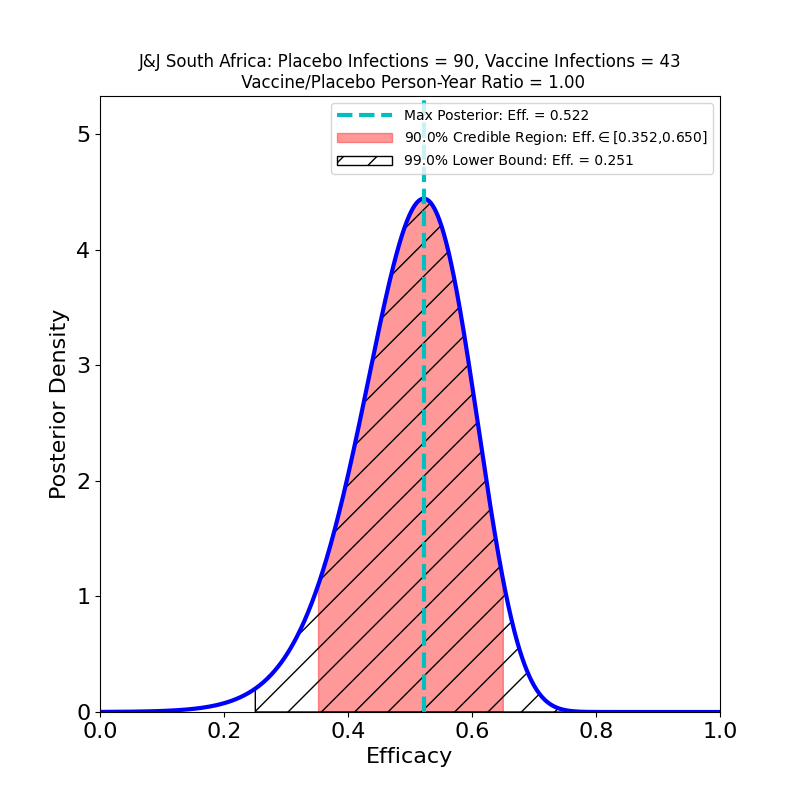
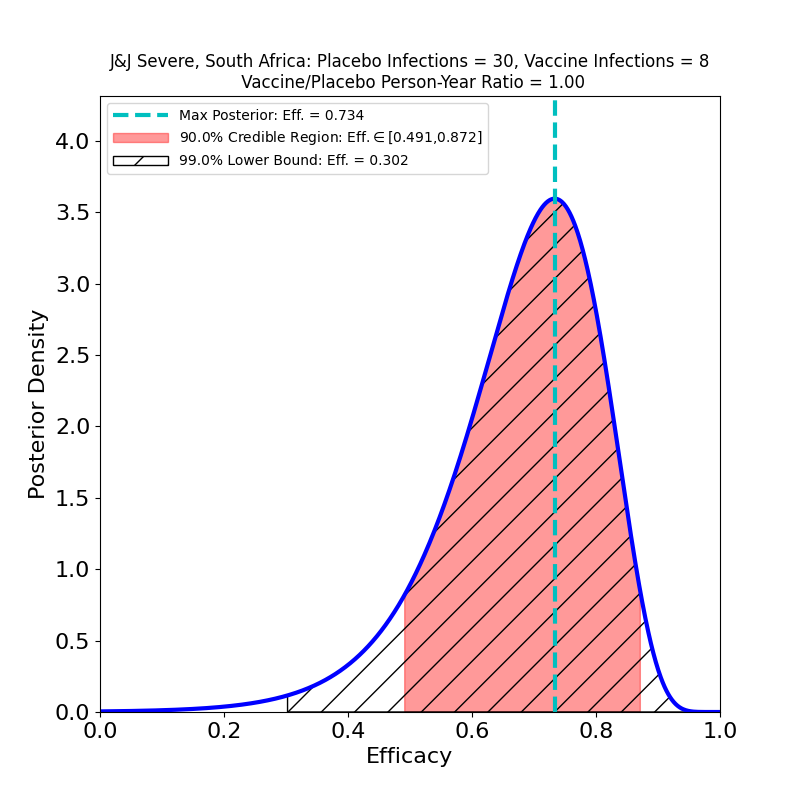
The bad news is from South Africa, where the B.1.351 variant constituted 94.5% of sequenced cases. Here the efficacy is clearly reduced (52% peak). The curve is wider, again due to lower subgroup numbers, so the actual efficacy could be a bit higher, but note that there’s more “pink” to the left of the peak than to the right. The area is proportional to probability, so unfortunately the smart bet would be that the actual efficacy is lower than 52%, rather than higher.
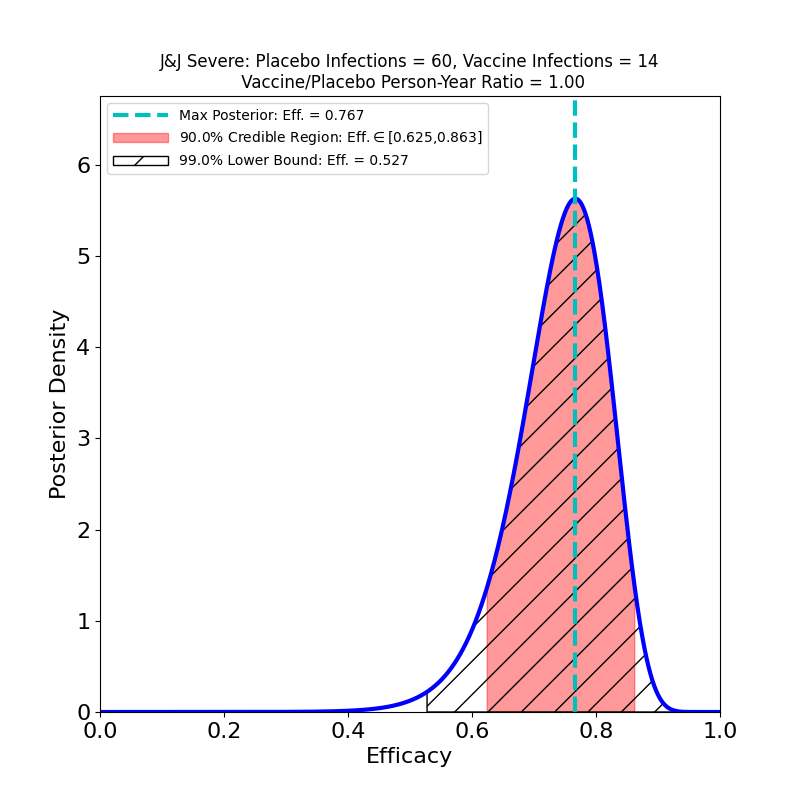
Now let’s take a look at protection against “Severe COVID-19”.
Here there’s some better news. The curves are all wider, because again the subset-of-a-subset numbers are lower, but there are some clearly improved trends. The efficacies are all systematically higher for the “Severe” disease sets than for “Moderate+Severe” sets. That is, in all countries, including South Africa, it appears that chances of contracting the severe form of the disease are appreciably lower for the vaccinated population. One should worry about the uncertainties due to low case numbers here, but again it is reassuring that in all cases the efficacy peaks move to higher values when only severe disease is counted.
It looks to me like a mixed-news situation. Protection against disease is clear except against the B.1.351 South African variant, and even here there seems at least to be reasonable protection against severe disease. On the other hand, protection against transmission (which is not technically evaluated in this study) is likely not great against B.1.351, since the vaccinated population will still have plenty of symptomatic virus-shedders, to say nothing of the asymptomatic cases.
To be fair to J&J, the Moderna and Pfizer/BioNTech vaccine trials did not confront variant subsets, so it is not possible to know with this kind of precision how badly affected their efficacies will be by the advent of B.1.351. Moreover, reportedly all the vaccine manufacturers believe that they can tweak their vaccines to target variants quickly, and the FDA has announced a speeded-up approval process for such re-tuned vaccines (similar to the annual flu vaccine approval process, which is highly streamlined).
My conclusion is that it is important to ship and jab as many doses as possible now, because one key factor in getting the epidemic under control is to stop the proliferation of variants, which appear in proportion to case numbers — the reason we’re suddenly beset by variants now, instead of in March, or August, is that case numbers during the winter wave eclipsed the cases in previous waves by a large factor. If we can get the cases down to the point that variants take much longer to develop, the vaccine-tuning process can control even the most transmissible variants. It’s a process, but there’s a good outcome at the end, I’m pretty sure.