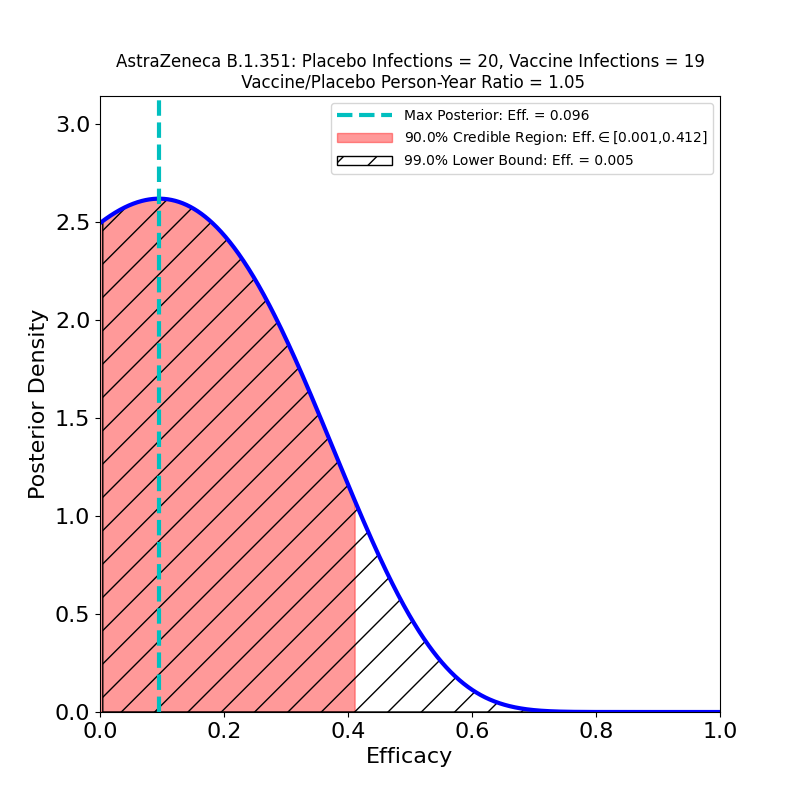
There’s a new journal article at the New England Journal of Medicine, detailing a Phase 1b/2 clinical trial of the AstraZeneca COVID-19 vaccine, conducted in South Africa. The principal thrust of the article is to gauge efficacy against the B.1.351 virus variant now prevalent in South Africa. The news is…not good:
This plot shows the efficacy of the vaccine, restricted to the sample of patients who developed “mild or moderate” COVID-19 (there were no “severe” cases in this small dataset) and were confirmed infected by the B.1.351 variant of SARS-CoV-2. Basically, there is no evidence of protective efficacy against the B.1.351 variant.
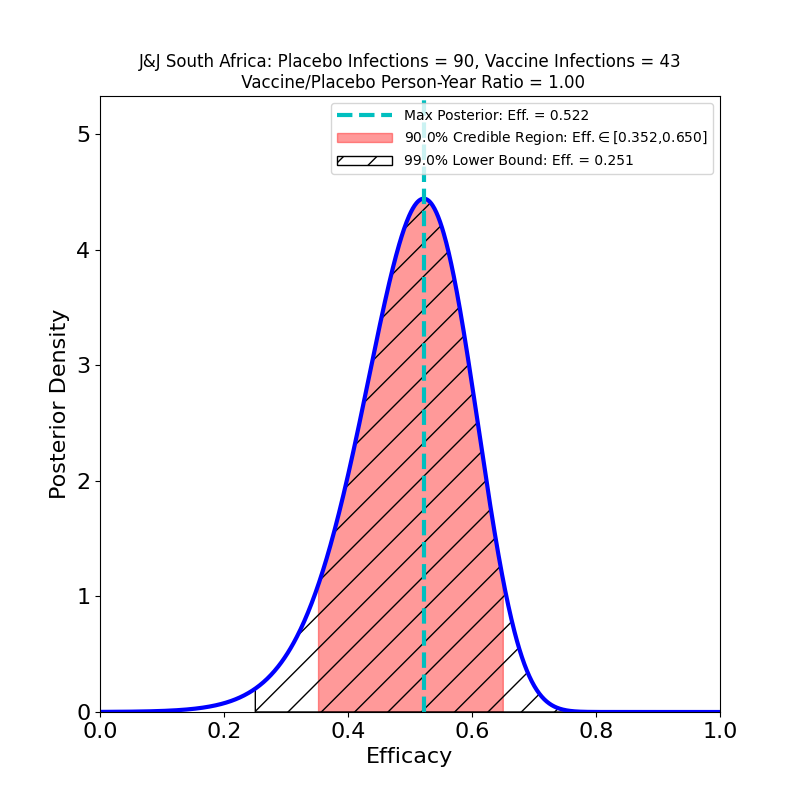
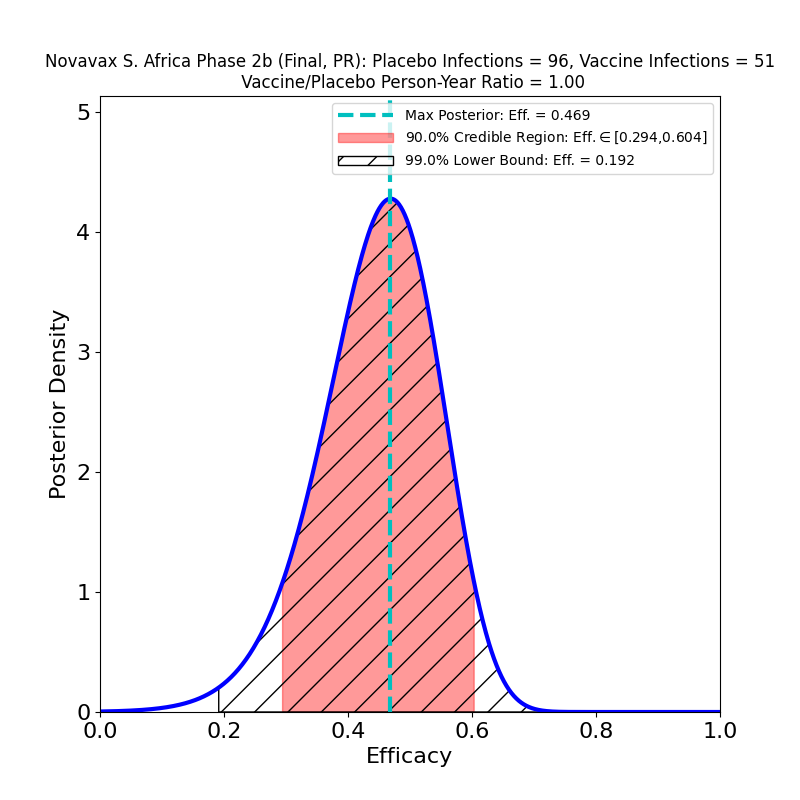
This is not to pile on to AstraZeneca, which is having a bad week anyway due to safety concerns. So far, no current COVID-19 vaccine has demonstrated acceptable efficacy against this particular variant (as opposed to the B.1.1.7 “UK” variant, or the P.1 “Brazil” variant). The Johnson & Johnson and Novavax vaccines also had disappointing performance against this variant. Collecting the three South Africa studies together for comparison, we see this:
None of these looks particularly good. Part of the reason the AZ efficacy plot looks much worse than those corresponding to the J&J and Novavax South Africa studies is that those studies had about 6 times more data, so the evidence for what protection their vaccines can offer is better than AZ’s evidence — the protection itself might be about the same. Also note that the J&J and Novavax studies did not single out B.1.351 cases, so there are about 5% “classic” SARS-CoV-2 cases in the above plots, and this necessarily improves their apparent efficacy with respect to the AstraZeneca result. Another confounding issue is that because of the incompetence of AstraZeneca’s vaccine manufacturing (which also led to the data issues that I discussed here), the administered doses were not all the same — the article states that vaccine recipients in the trial received “…a 0.33-to-0.5-ml dose (depending on the lot) of the ChAdOx1 nCoV-19 vaccine”, which means they don’t even have a well-controlled idea of how much vaccine they administered in this study.
Note also there is in vitro evidence that the Pfizer and Moderna vaccines will offer at best weak protection against B.1.351 (see here for Pfizer and here for Moderna).
So the story of vaccine-mediated resistance to this variant remains the same as I discussed in this post. At the moment, there is weak evidence for protection against severe disease caused by B.1.351, and no evidence at all for protection against transmission. We’re still on-track for an urgent program of B.1.351-specific vaccine booster shots (Moderna has one already in trials) as soon as they can be developed, approved, and produced. In my opinion the need for such boosters will be clear by late Summer 2021, and getting urgent by Fall.