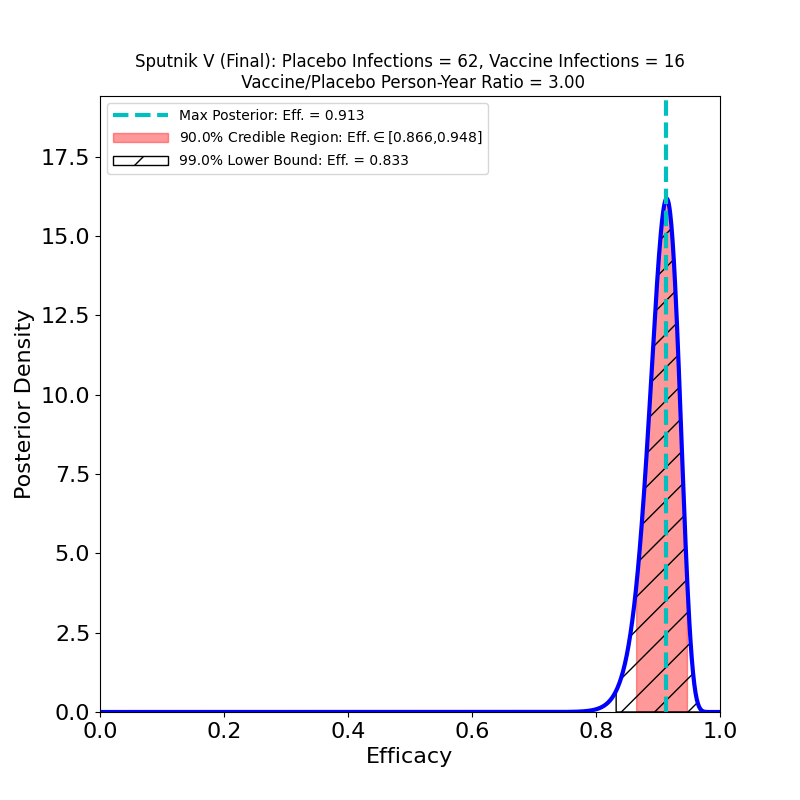
This is an adenovirus-vector vaccine with a 2-dose schedule — the two doses use different adenoviruses “…to overcome any pre-existing adenovirus immunity in the population.” The Phase 3 results are reported in this article. One clever thing about this study is that the placebo group is only 1/3 the size of the vaccine group, which should increase the sensitivity of the study to the efficacy signal, assuming sufficient placebo infections occur (not a difficulty in a raging epidemic :-( ).
The overall efficacy appears to be very good — a bit lower than Moderna’s and Pfizer’s vaccines, but higher than AstraZeneca’s vaccine. Since AZ’s vaccine is also adenovirus-mediated, it is interesting to compare the two. Oddly, Sputnik V obtains its efficacy without a reduced first dose, despite AZ’s reduced efficacy with two standard doses. Perhaps the dual adenovirus vector helps avoid the pitfall that befalls the SD-SD case in AZ’s study.
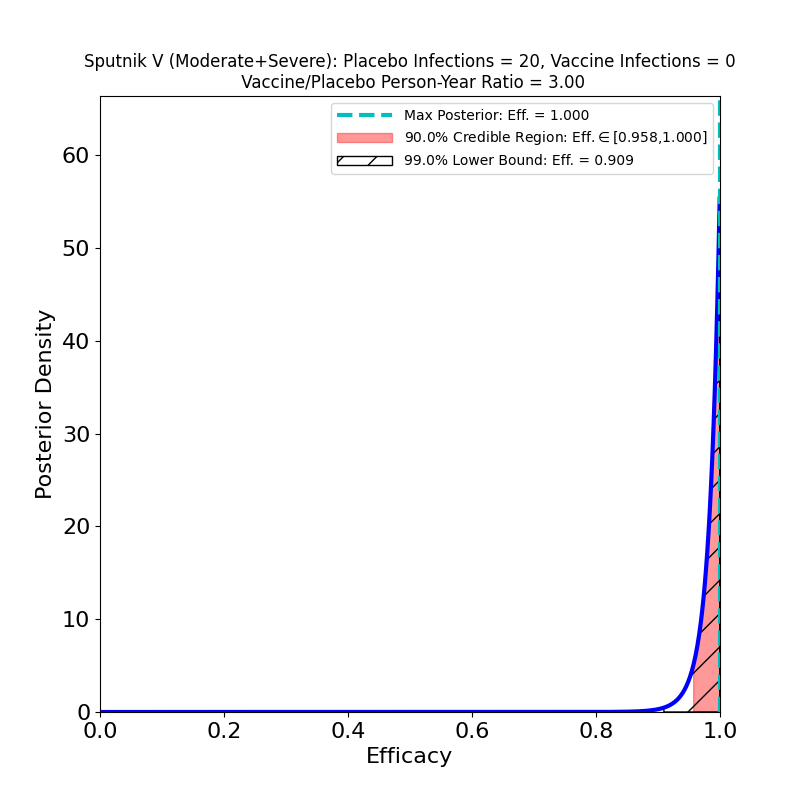
Also, the protection against “moderate + severe” symptoms is clearly excellent. The clever choice of placebo-to-vaccine group size really helps here. There are 20 “moderate+severe” cases, which is fewer than the 30 in Moderna’s study, but the power of those cases is amplified by the study design, so that the posterior density of the Sputnik V “moderate+severe” cases is more concentrated than is the case for the Moderna “severe” cases.
I would caution that it is difficult to compare different vaccines for this kind of protection, as the subset definitions of “severe”, “moderate” etc. are completely study-dependent. One would really have to read the journal articles carefully to understand the extent to which these categories can be compared across studies. I don’t feel qualified to do this myself, so I’ll just issue the caveat.