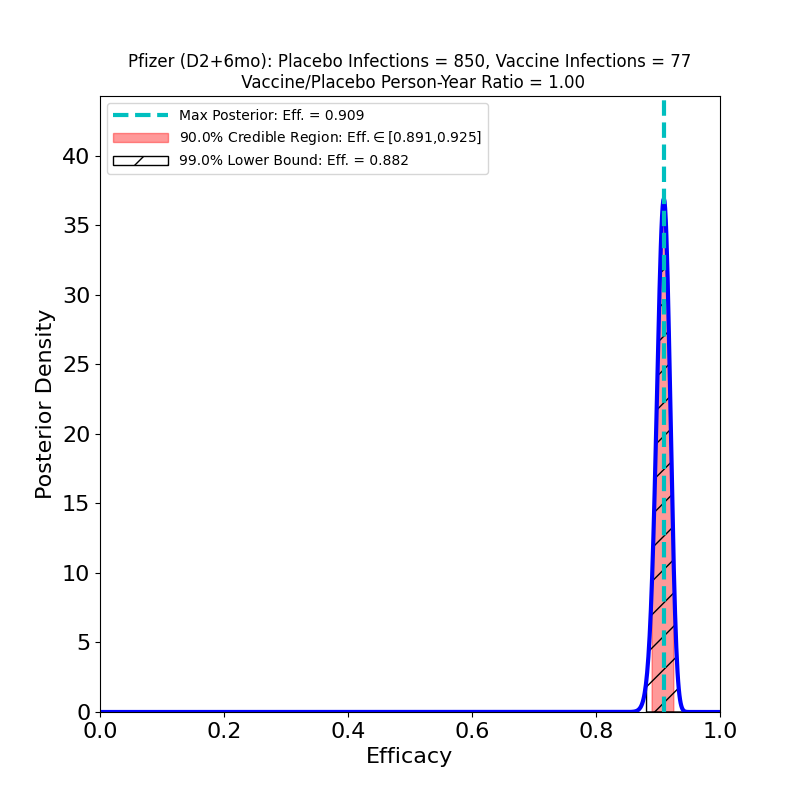
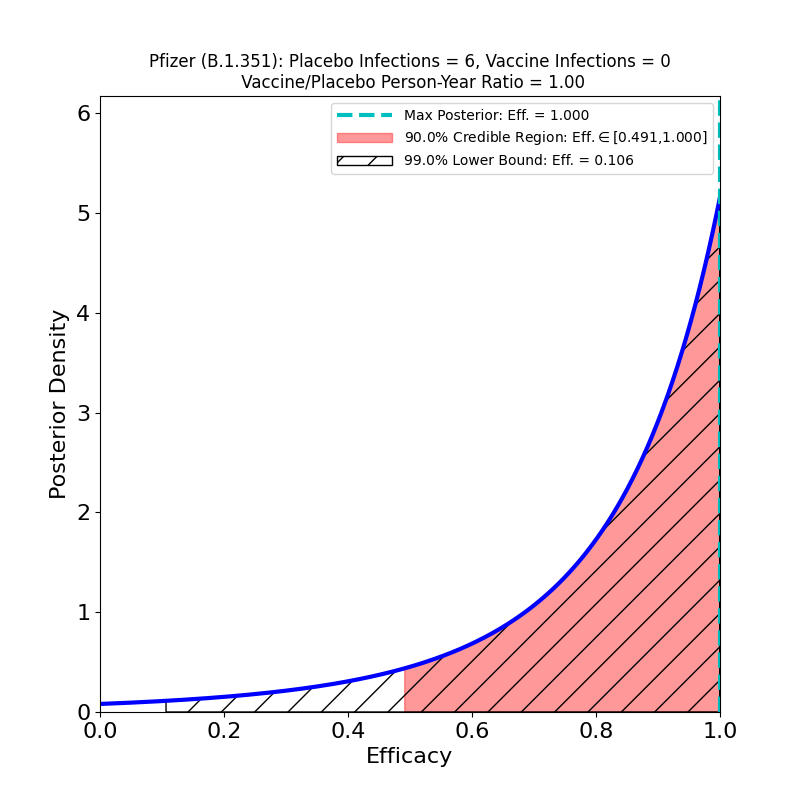
Another press release by Pfizer, this one with results from their main phase 3 trial (46,307 participants). They have followed up participants for 6 months post shot-2. The press release has some slices of data, which will certainly be released in more detail at the time of their application for full FDA approval — and presumably when they submit a journal article.
Here are the two things that caught my eye:
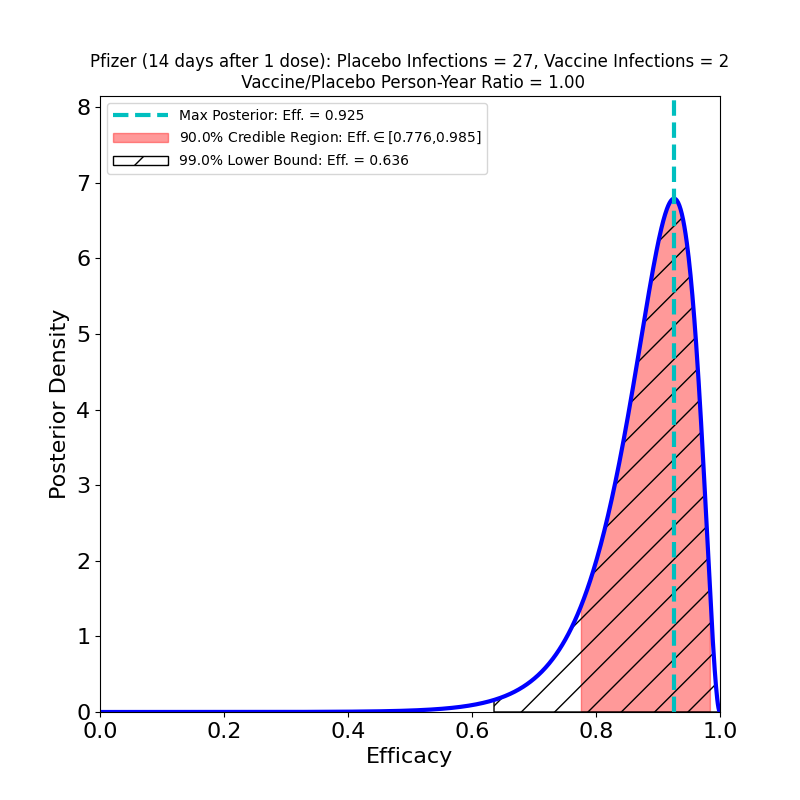
On the left we see more confirmation of what we knew: the superb efficacy of the Pfizer/BioNTech COVID-19 vaccine. There is now so much data that the uncertainty on the efficacy is tiny. The peak posterior probability estimate in the plot is 91%. The result is basically consistent with the previous results. It’s worth remembering that the original targets for vaccine efficacies when the development effort began in early 2020 was “anything over 50%.” The fact that this generation of vaccines is ringing the bell in the 70-90% range is really an astonishing feat.
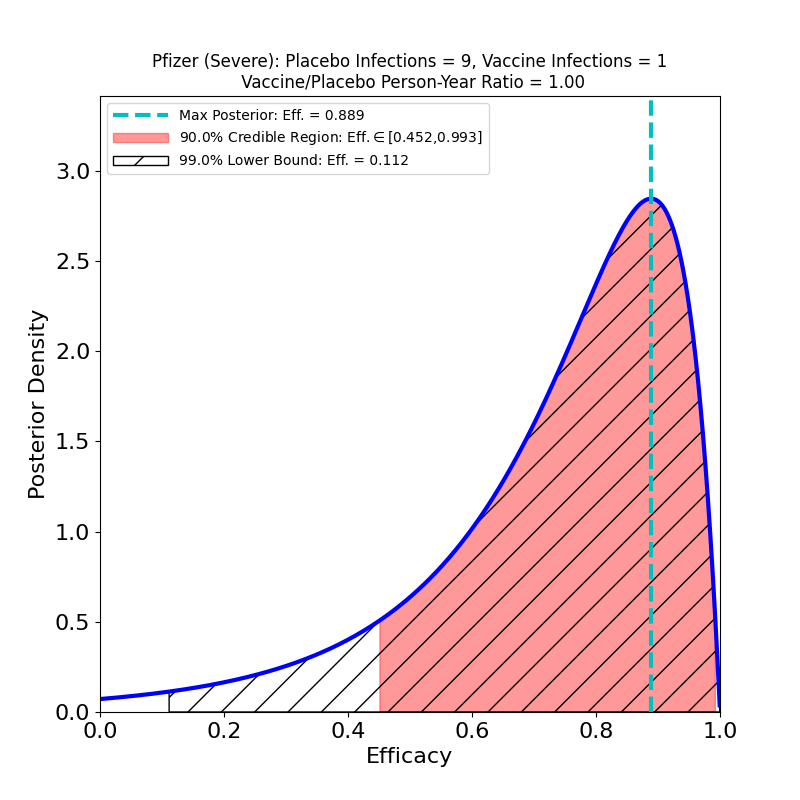
Now to the right-hand panel. The trial has a South Africa arm (well, at 800 participants, more like a “finger”). Of these, 9 placebo-group participants became infected, and zero vaccine group-participants. Of the 9 placebo-group participants, 6 were confirmed to have been infected by the B.1.351 lineage. If you have 6 placebo infections, and zero vaccine infections, you get the plot on the right hand side. This is what the flacks who work for Pfizer’s PR department have to say about this:
In South Africa, where the B.1.351 lineage is prevalent and 800 participants were enrolled, nine cases of COVID-19 were observed, all in the placebo group, indicating vaccine efficacy of 100% (95% CI, [53.5, 100.0]). In an exploratory analysis, the nine strains were sequenced and six of the nine were confirmed to be of the B.1.351 lineage. These data support previous results from immunogenicity studies demonstrating that BNT162b2 induced a robust neutralizing antibody response to the B1.351 variant, and although lower than to the wild-type strain, it does not appear to affect the high observed efficacy against this variant.i
OK, now look at that plot. Does that say “100% efficacy against B.1.351” to you? Because to me it says “we got bupkus for evidence, since we had too few people in South Africa participating in this study.” The 90% credible region starts at 50% efficacy!
You should also be aware that the stuff about “robust neutralizing antibody response” is based on this paper, which however showed that in-vitro neutralization of B.1.351 required far more concentrated vaccine-induced antibodies than were required for other variants — I cannot understand how the how one could possibly characterize that result as “robust”. In any event, its a wet lab result, not a clinical trial. We still don’t have any quality evidence about the performance of the mRNA vaccines against the B.1.351 variant. And that has me very worried.