Preliminary results from the phase 3 clinical trial of Bharat Biotech’s COVAXIN vaccine are now available from a press release. The vaccine is a “classic” inactivated virus design. Here’s the Bayesian analysis of the released data:
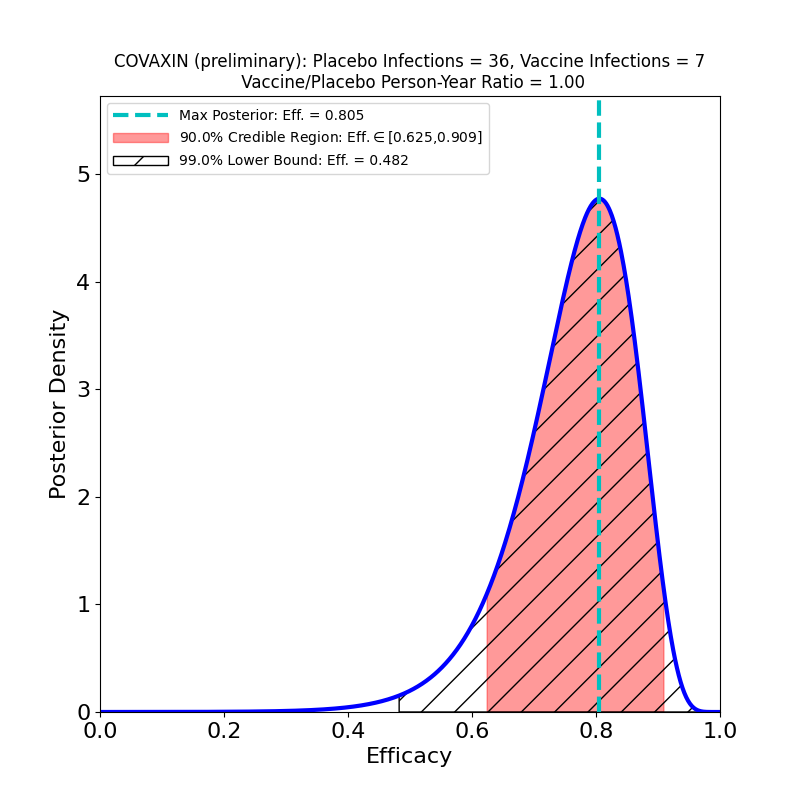
So far so good. The numbers are too low at the moment to constrain the efficacy very tightly, but with a 90% credible region lower bound at 63% and a peak probability density at 80%, it looks very promising — comparable to the AstraZeneca LD+SD result (which was from a full clinical trial!). I’ll revisit COVAXIN when the full clinical trial data is released.
I would invite commentary by experts on how easy it will be to re-target this vaccine at variants that might resist the immunity it produces (such as, in all likelihood, the South Africa B.1.351 variant). In particular, can variant-targeting boosters be manufactured quickly at large scales? This is going to be a key question going forwards, as I discussed in this post.