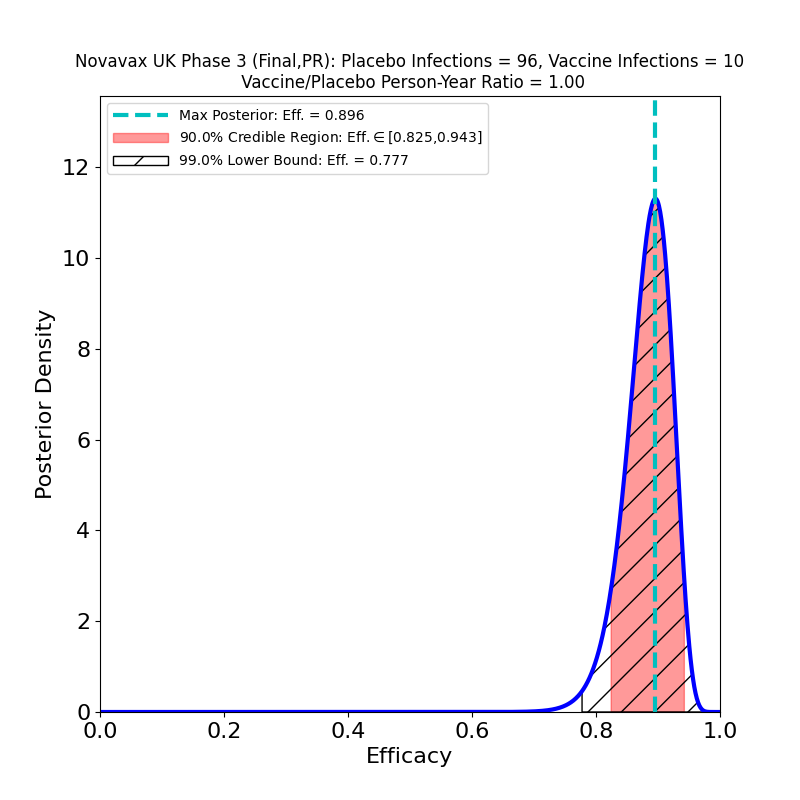
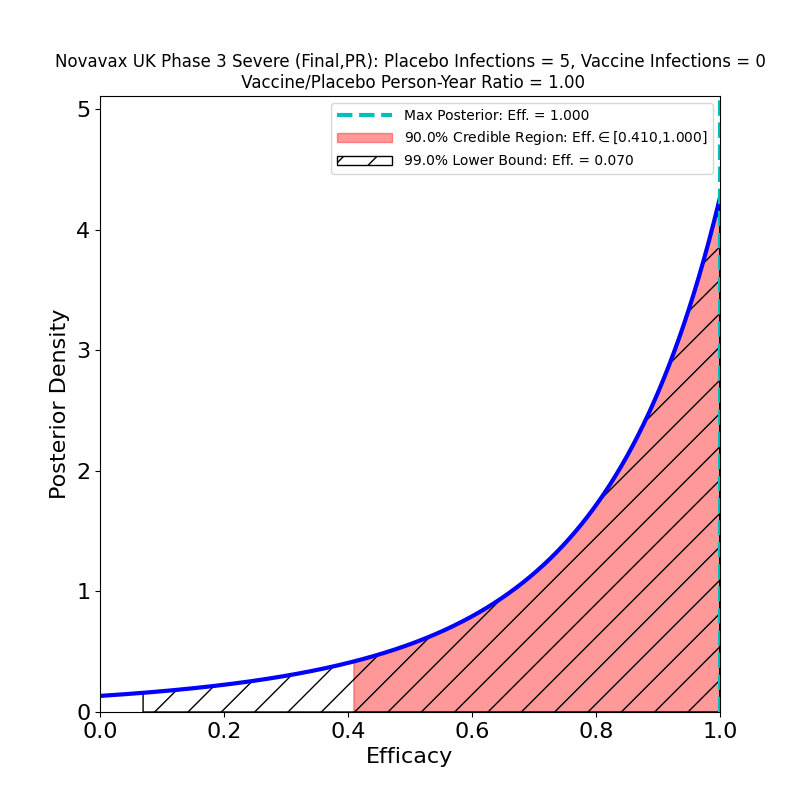
Novavax issued a press release today with final results of a second phase 3 trial — a separate one from the one that the company conducted in the UK, which I discussed in this post. It appears to be a larger study (about 30,000 participants, twice the 15,000 of the UK study) and randomized 2:1 vaccine-to-placebo to increase the efficacy signal. I’m not certain of the reason for the study duplication, but there are some undoubted benefits that have resulted from it: having been conducted later in the epidemic than the trials of earlier vaccines, this study saw more “Variants of Interest” (VOI) and “Variants of Concern” (VOC) as defined by the CDC, and was able to target them specifically in assessing efficacy. The result is surprisingly good news.
Here are the results:
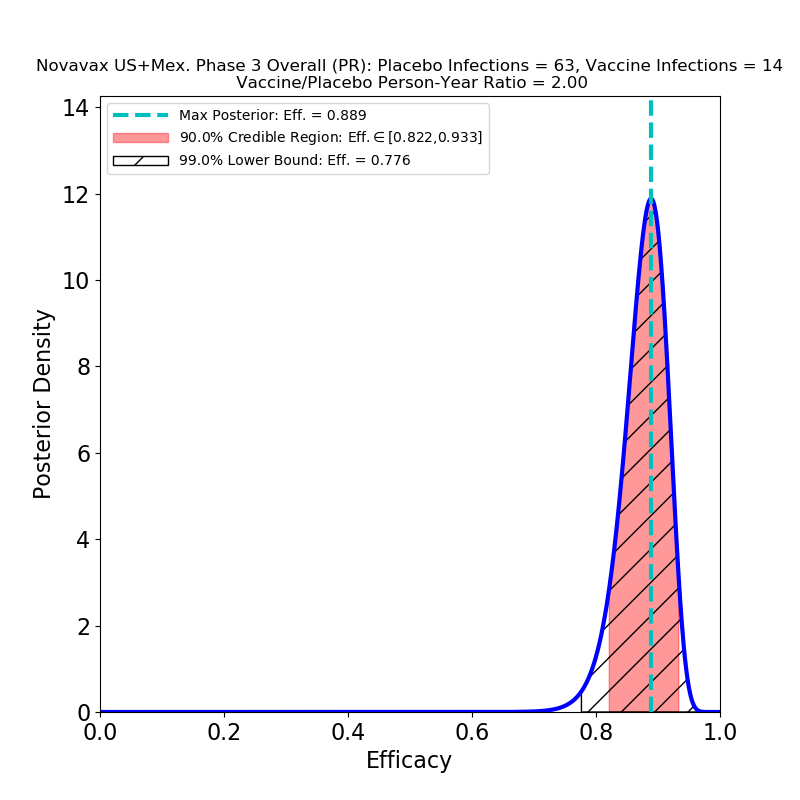
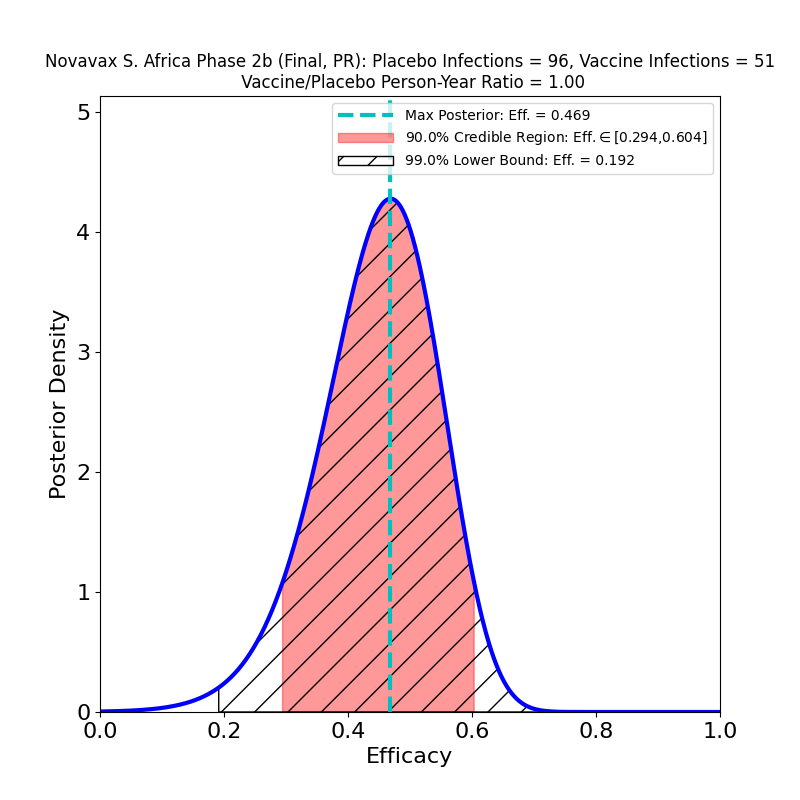
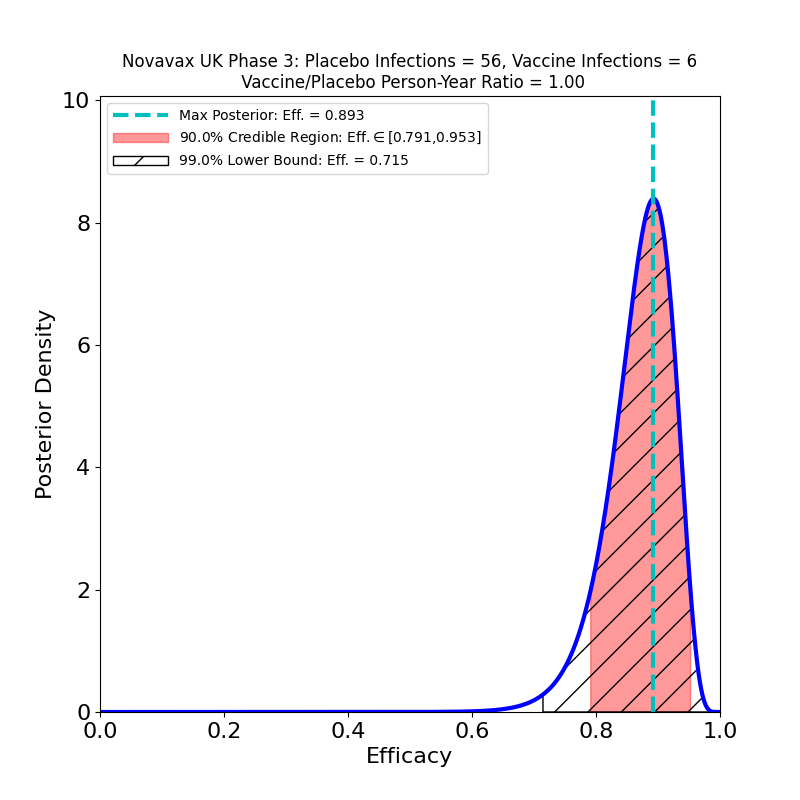
The first point is that this vaccine is in the same sort of efficacy category as the Pfizer/BioNTech and Moderna vaccines — perhaps a few percent lower, but still this is spectacular performance, with a peak at 89% (Novavax claims 90.4%, I’m not sure what the source of the small discrepancy is, but it’s probably not important). Given that this is a vaccine that stores at 2-8 degrees C, we now have another important tool for large-scale global vaccination programs, and none too soon.
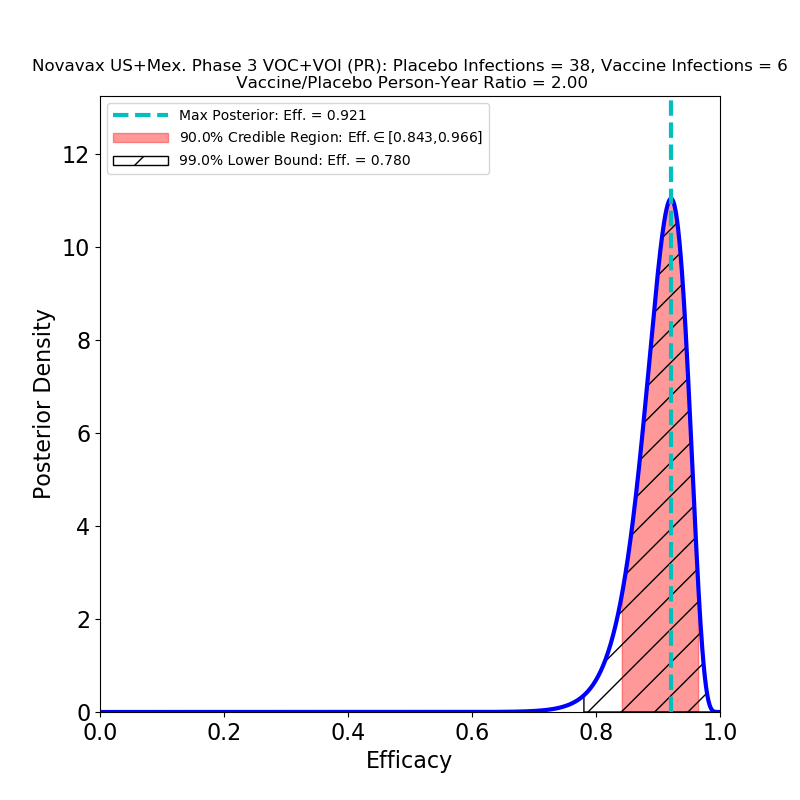
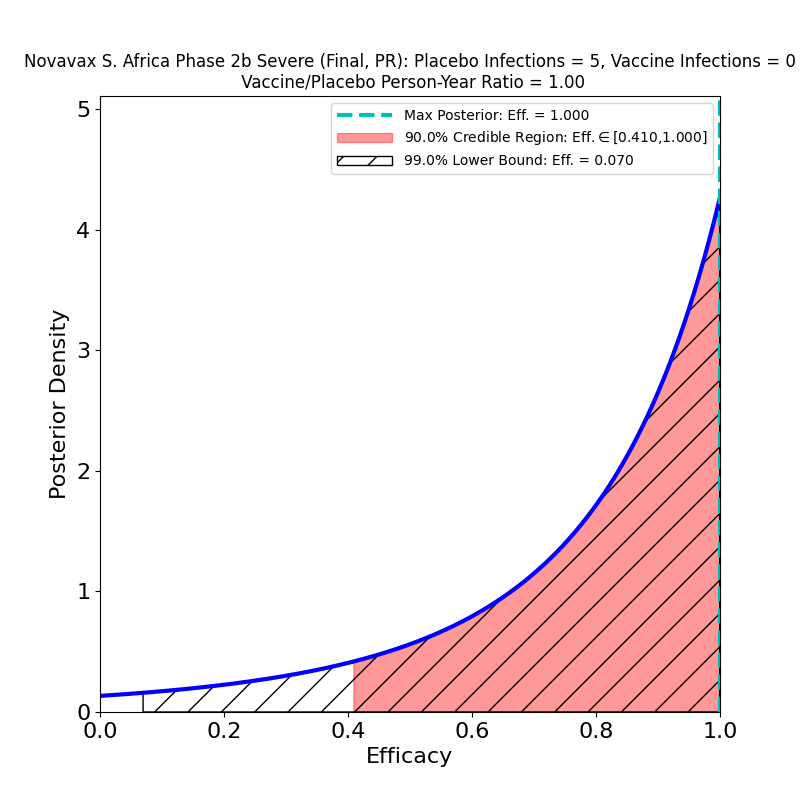
The second point is performance against variants. Compare the right panel to the left: This is not what I expected, at all. There is no significant decrease in the efficacy of the vaccine against variants. Which could mean broad protection against mutated strains of SARS-CoV-2. Of course, the press release does not specify which variants are implicated, and the lumping together of several mutations may mask some bad news that will only be discernible later.
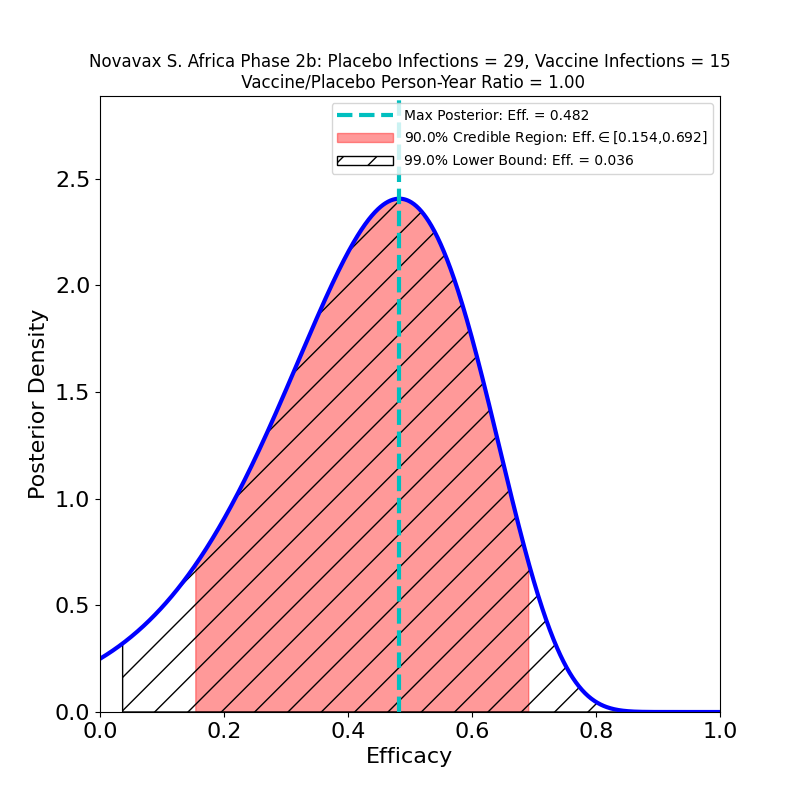
Recall that a Variant of Concern is defined as one “…for which there is evidence of an increase in transmissibility, more severe disease, significant reduction in neutralization by antibodies generated during previous infection or vaccination, reduced effectiveness of treatments or vaccines, or diagnostic detection failures.” The B.1.1.7 (“UK”) variant is a higher-transmissibility VoC, but it seems to be effectively blocked by the present crop of vaccines. On the other hand, the B.1.351 (“South Africa”) variant is a reduced-vaccine-effectiveness VoC, since it seems to have some trick that reduces vaccine-induced immunity, to a poorly-known extent that depends on the type of vaccine — in fact, Novavax’ own Phase 2b South Africa trial took a major hit from this variant. Obviously, lumping these two VoCs together in a single study would not be a useful thing to do, since might conceal some serious trouble to be expected later on. If, for example, the mix of VoCs in the US and Mexico is currently not rich in B1.351, but the proportion of that variant grows in the next few months, it is possible that we could see the efficacy of this vaccine decline.
On balance, however, I would say the story here is guardedly positive. As usual, more information is needed than is supplied in a press release.