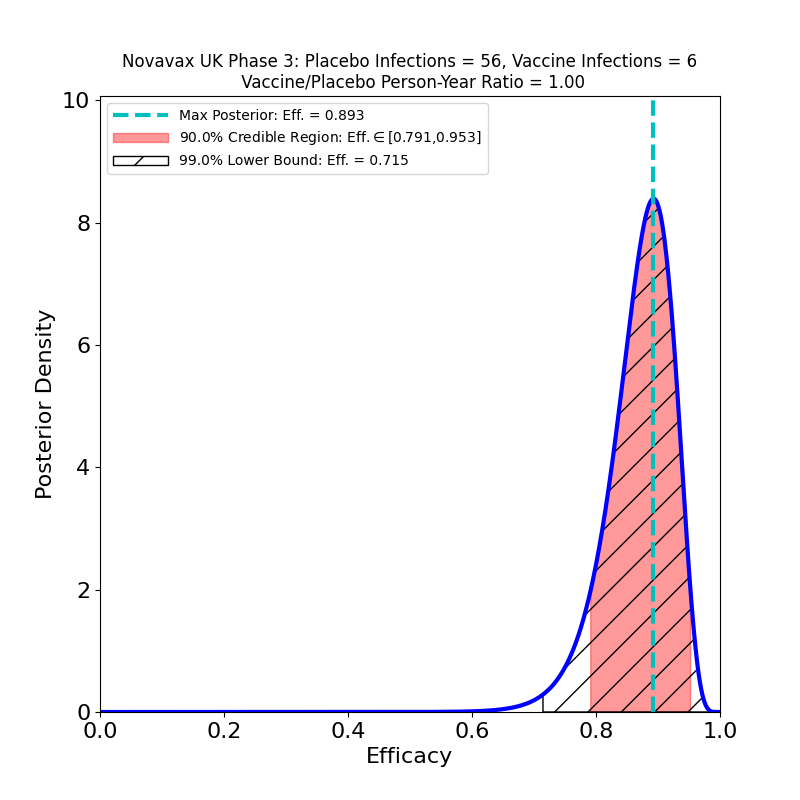
These plots result from the data reported in a Novavax corporate press release issued on 28 January 2021. I’ll update them if more detailed and/or authoritative information becomes available.
Novavax reported results from two clinical trials: a Phase 3 trial performed in the UK, and a Phase 2b trial performed in South Africa. The press release does not state the dose schedule, but from information at clinicaltrials.gov here and here it appears to be a 2-dose schedule, with doses 21 days apart. The South African trial was performed under conditions of high prevalence of a new variant of the SARS-CoV-2 variant. Here are the plots:
In the UK, against SARS-CoV-2 “classic”, the efficacy is as impressive as the other principal vaccines (e.g. Moderna, Pfizer/BioNTech, AstraZeneca, Sputnik V). The peak at 89% is certainly very encouraging news.
The South African trial results are worrisome, however. The numbers are still small (this is a Phase 2 trial), but the peak efficacy against the South African variant is below 50%. It is not clear to me why the press release claims that 15 vaccine cases and 29 placebo cases results in an effectiveness estimate of “60%”, unless they chose a larger size for the vaccine group than for the placebo group — the press release doesn’t contain the necessary detail. I’ll be revisiting this one when the company submits a briefing document to the FDA EUA committee, or when they publish a journal article.
In any event, it seems clear that (a) the efficacy against the South African variant is considerably lower than against the “classic” SARS-CoV-2, and (b) the study numbers are not yet large enough to ascertain how much lower — the 90% credible region goes from 69% (only a little lower than the UK estimate) to 15% (basically useless). It seems clear that this vaccine — and probably all the other ones — will have to be updated soon to fight new variants.
Updated 5 February 2021: Dan, in the comments, points out that the 60% efficacy claimed in the press release corresponds to efficacy in HIV-negative participants. I should learn to read the nuances in press releases more carefully…
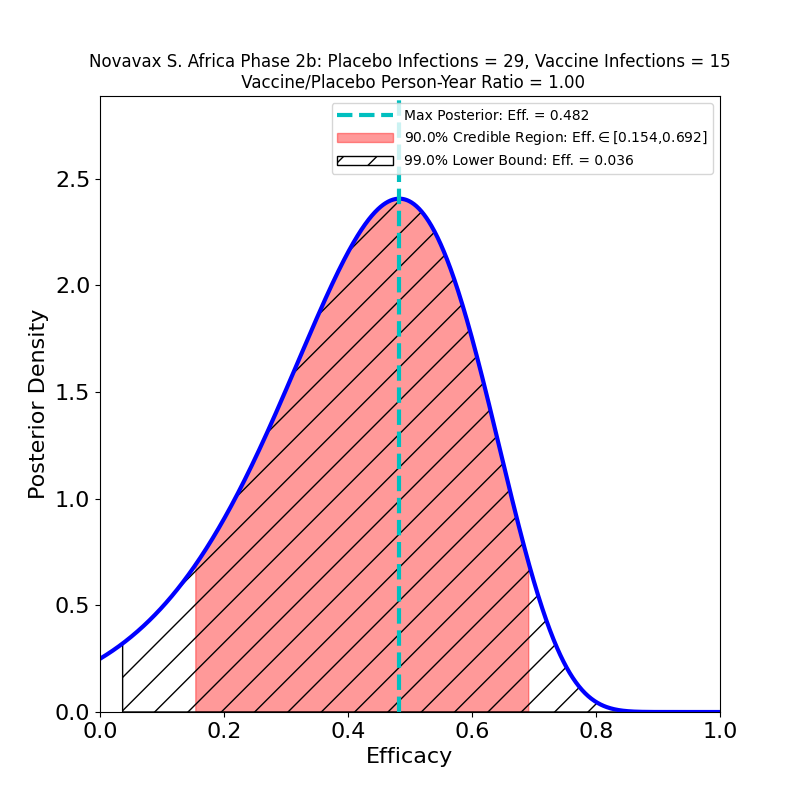
The 60% figure comes from the efficacy in HIV-negative participants.
Ah. Thanks for the clarification. I’ll edit the post accordingly.